Oil In Water Vs Vater in Oil Emulsions
In 2015 Grand View Research estimated the worth of global food emulsifier market to be approximately $4.3 billion. Mankind has been using emulsions since the beginning of times and the first food-based emulsion is none other than milk itself. However, the first classification of emulsions was published in a 1910 research article by Wolfgang Ostwald.
Not only margarine, butter, ice-cream are emulsions but creams, lotions and liniments are also classified as emulsions thereby encompassing food, pharmaceuticals and cosmetic industries within its scope.
What are emulsions? Differences between oil in water and water in oil emulsion and many other emulsions’ related information has been discussed in this blog OIL IN WATER VS WATER IN OIL EMULSIONS. Without further delay lets delve deeper into the science of emulsions.
1.What are emulsions?
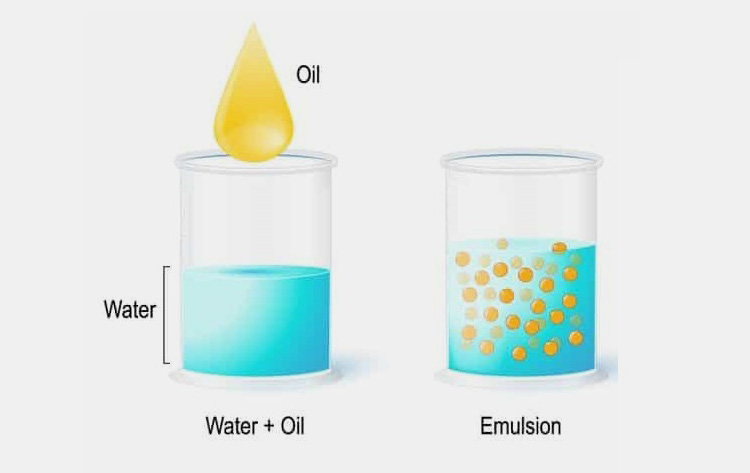
When two immiscible (or partially miscible) liquids are brought together in the presence of an emulsifier they form a dispersed system that is termed as an emulsion.
In this type of system one liquid is dispersed as small droplets or globules, in to other, having a usual size of 0.1 to 10µm.

The dispersed liquid is called as internal or discontinuous phase whereas dispersion medium is termed as external or continuous phase.
2.Classification of emulsions?
Some common examples of emulsions are creams, lotions, liniments, butter, ice-cream etc. that means there are various types emulsions and logically more than one way to classify them. An emulsion can be classified based on either dispersed medium or size of droplets or stability of the entire system.
Based on dispersed medium:
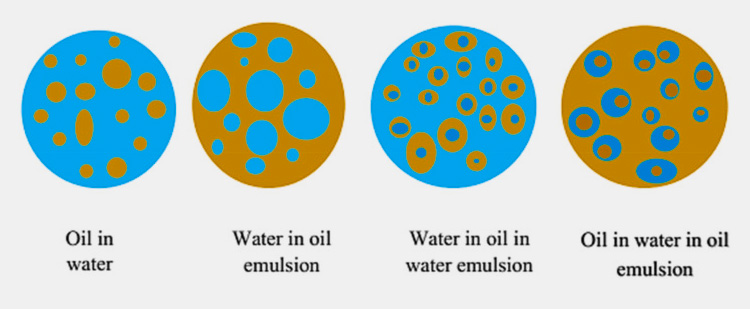
Oil in water (O/W): an emulsion where oil is the internal phase and water is the external one. For example, mayonnaise and Hollandaise sauce.
Water in oil (W/O): in waterthe oil emulsions water globules (internal phase) are dispersed through dispersion medium (external phase) which in this case is the oil. For example, butter and margarine.
Water in oil in water (W/O/W) or oil in water in oil (O/W/O): these are complex emulsions can also be called as emulsion within an emulsion. In this type of complex system oil in water or water in oil emulsion is dispersed in another medium which happens to be either water or oil.
For example, novel carrier in pharmaceutical and cosmetic industries i.e. prolonged release formulations and skin moisturizers.
Based on droplet size
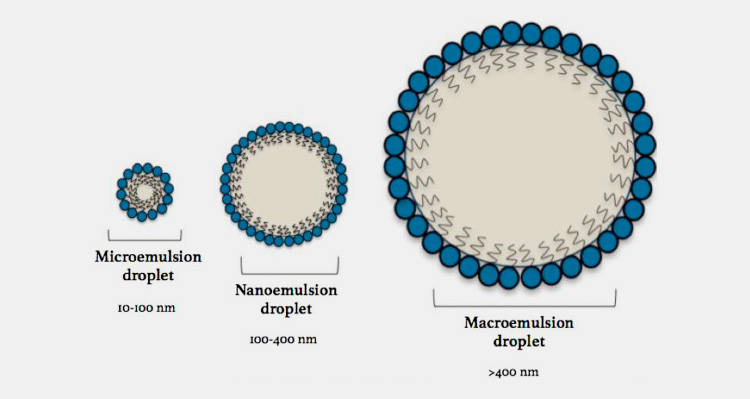
Macroemulsions: droplet size of the internal phase is 200 µm – 50 mm. Thermodynamically unstable, macro-emulsions tend to revert back to their original form however, having low kinetic energy they remain stable from a macroscopic outlook. Examples are alcohol and water, silicone and water etc.
Microemulsions: microemulsions are thermodynamically stable and have an internal phase droplet size of 10 – 200 µm.
Based on stability of emulsions
Temporary emulsion: A mixture of oil and vinegar is termed as temporary emulsion because it separates with an hour and forms again with little agitation or shaking.
Semi-permanent emulsions: Hollandaise sauce is an example of semi-permanent emulsions that has a stability of few hours. It is a mixture of egg yolks and clarified butter.
Permanent emulsions: These emulsions remain stable for extended periods. Mayonnaise a mixture of egg yolks and oil is an edible example of permanent emulsions.
3.How are emulsions formed?
Emulsions are a combination of two immiscible liquids generally stabilized by emulsifier that can be classified in a number of ways and can be formed by a number of methods.
Formulation of emulsions is quite easy and can be carried out in a home-based setting. Some of the methods of making an emulsion are given below.
General method
Generally, emulsions are formed when oil and water phases are agitated in the presence of an emulsifier. For example, when oil is added to egg yolks while blending vigorously mayonnaise is formed. Water content promotes emulsification on coming in contact with oil whereas lecithin and protein in yolks act as emulsifier.
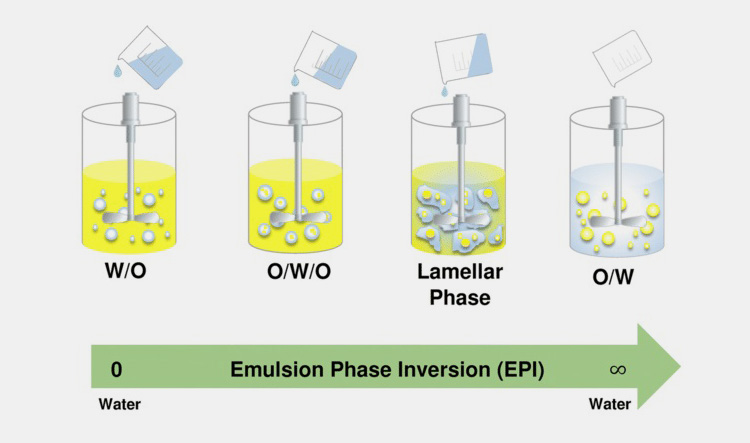
Phase inversion
In simple emulsions whether oil in water or water in oil, phase inversion can be achieved by changing the affinity of emulsifier from one phase to other. Another way of achieving phase inversion is to change the water to oil ratio. The former process is known as transitional phase inversion where as latter is called as catastrophic phase inversion.
Dry and wet gum method
Dry gum method is used for extemporaneous preparations in this method emulsifier is mixed with oil phase which is then mixed with water phase.
In wet gum method emulsifier is mixed with water to form a mucilage which is then added to oil phase.
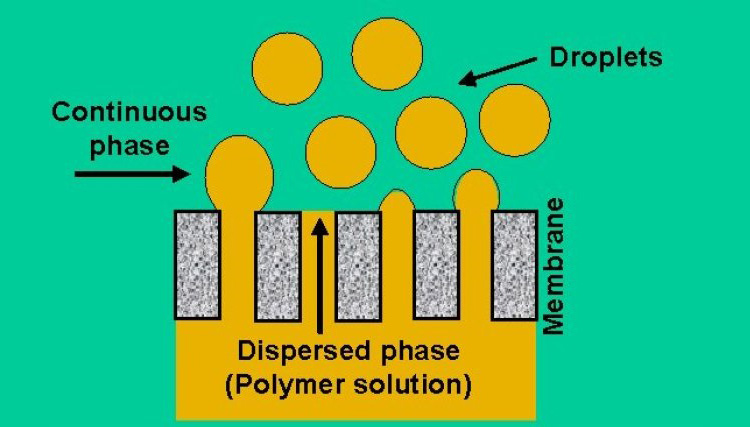
Membrane emulsification
It’s a relatively novel technique first developed by Nakashima and Shimizu in 1980’s. In membrane emulsification dispersed phase is converted to droplets of required size by passing it though a membrane and incorporated into the continuous phase.
Membrane emulsification can be achieved by two different processes one is called pre-mix membrane emulsification and the other one is known as cross flow membrane emulsification. Generally cross flow membrane emulsification is more commonly used between the two processes.
4.What are the theories of emulsification?
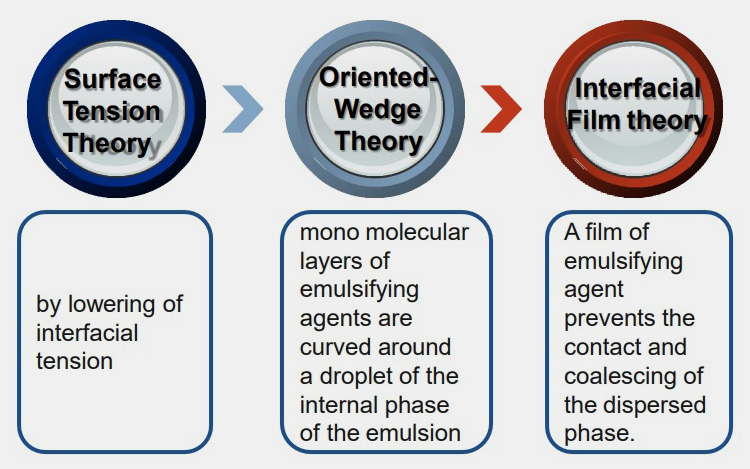
There are three theories that have been postulated about the process of emulsification namely;
Surface tension theory
According to this theory emulsions are formed when interfacial tension between two immiscible liquid is reduced by a surfactant; leading to a decrease in the attractive forces between similar and repulsive forces between opposite molecules.
Oriented wedge theory
A surfactant is a substance that possesses both hydrophobic (non-polar) and hydrophilic (polar) part. This theory deals with the shrinkage of either non-polar or polar part of surfactant based on their affinity, leading to the formation of emulsions.
Interfacial film theory
This theory states that emulsions are formed when a thin layer of surfactant adsorbs on the internal phase (globules) thereby preventing direct contact of the dispersed phase with dispersion medium subsequently leading to reduces coalescence between both phases.
5.Difference between Oil in water and water in oil emulsions; a complete overview
| Oil in water (O/W) | Water in oil (W/O) |
| Dispersed phase is oil whereas continuous phase is water. | Dispersed phase is water and dispersion medium is oil. |
| Commonly used in moisturizing products and edible products. Like moisturizers and steroidal products. | Used in extemporaneous preparations that prevent moisture absorption. For example, cold creams and sunscreen. |
| Non-greasy as compared to W/O emulsion. | Greasy and insoluble in water. |
| Gives positive conductivity test. | Gives negative conductivity test. |
| Water soluble drugs release quickly. | Oil soluble drugs release quickly in W/O emulsion. |
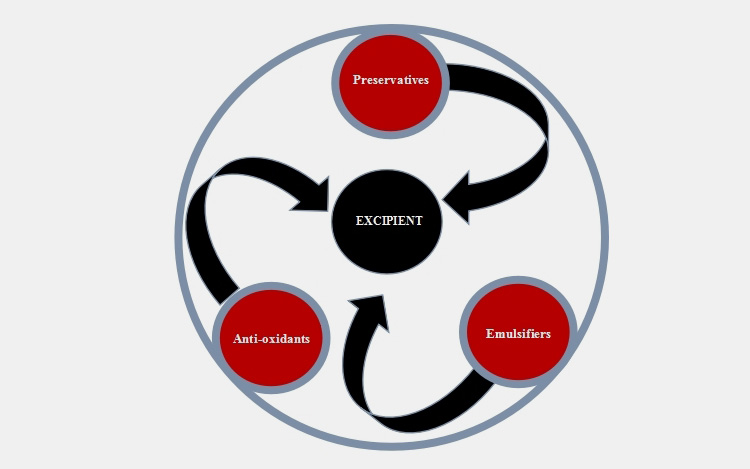
6.Excipient involved in formulating pharmaceutical emulsions.
Other than active ingredients, water and oil phases the excipient involved in formulating include following;
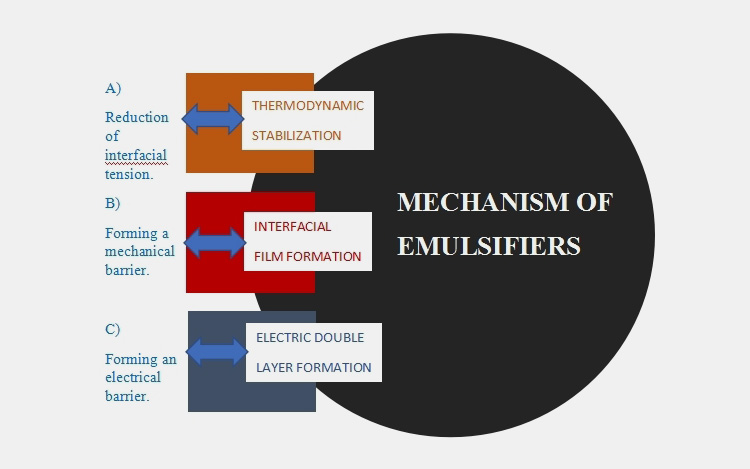
7.Associated mechanism of emulsifying agents.
An emulsifier works via three different mechanisms namely;
8.Equipment involved in manufacturing pharmaceutical emulsions.
| EQUIPMENT | DESCRIPTION | PICTURE |
| Wedgwood / porcelain mortars and pestles | Used at laboratory scale or for compounding of volatile/fixed oils. |  |
| Propeller mixers | Used for both small and large scales, propeller mixers are used for manufacturing low viscosity emulsions. |  |
| Turbine mixers | High viscosity emulsions are manufactures using turbine mixers. |  |
| Homogenizers | Homogenizers are the most commonly used equipment for manufacturing of emulsions. |  |
| Ultrasonifiers | Used for laboratory scale manufacturing of extremely low particle size emulsions. |  |
| Colloid mills | Colloid mills are used on regular basis for high viscosity emulsions. This equipment is ideal for solid milling and dispersion of poorly wetable suspensions. |  |
| Silverson mixer | High shear is required for smaller droplets of discontinuous phase. Silverson mixer is ideally suited for manufacturing 2 – 5 µm size droplets. |  |
| Micro-fluidizers | Microfluidizers manufacture nano-emulsions having droplets size in nanometers that are stable and functional. |  |
9.Stability problems associated with emulsions.
The most important aspect of a pharmaceutical emulsion is the stability i.e. the product maintains its form, color, odor and elegance during its intended shelf life. Some major stability problems associated with emulsion are given below;
| Problem | Description | Reason |
| Flocculation | Molecules of dispersed phase come closer to each other. | Non-uniform particle size distribution.
Reduced viscosity of the dispersion medium. Attractive charges between the molecules. |
| Coalescence | Dispersed phase molecules merge together, characterized by increased globule size and reduced number of globules. | Quantity of emulsifier.
Incompatibility of emulsifier. Emulsifier portioning. |
| Creaming | Dispersed phase either concentrates on the upper or lower part of the emulsion. | Viscosity difference between two phases.
Size of the globules. Reduced viscosity of the dispersion medium. |
| Phase inversion | Unintentional change of O/W emulsion to W/O emulsion or vice versa. | Over dilution with the continuous phase.
Improper temperature conditions during manufacturing. |
10.How to determine the nature of emulsion?
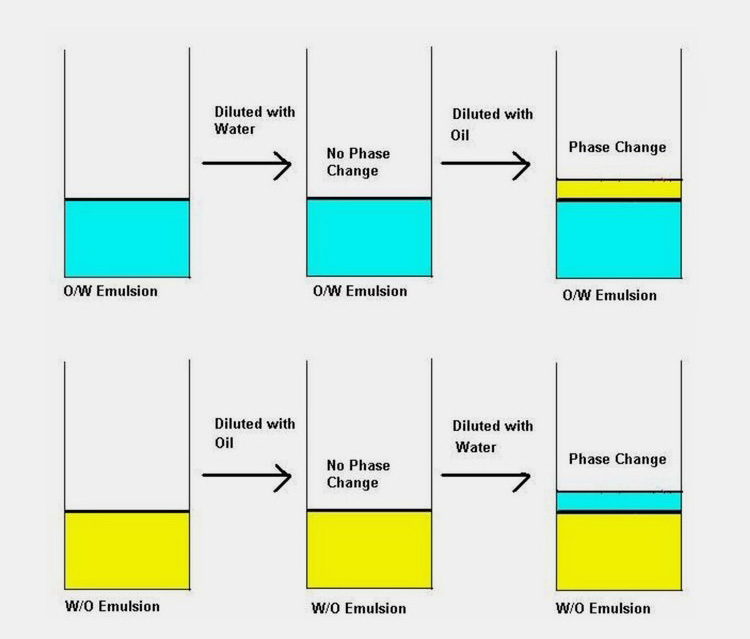
As you are now well aware that emulsions are generally of two types O/W and W/O excluding the complex ones; the next question would be how you can determine whether the emulsion is oil based or water based.
Usually the type of emulsion is determined by relative phase volume, but the recipe will be with the product developer, you would require to run certain tests. There are few methods that you can employ to check the nature of emulsion such as;
Miscibility tests
If emulsion is O/W type addition of water will not destabilize it where as if you add oil to O/W emulsion separation of both phases will take place. Same is the case with W/O type i.e. addition of continuous phase (oil) will not destabilize the emulsion were as water will cause stability issues.
Electrical conductivity
Water is a good conductor of electricity O/W emulsion gives positive results in conductivity tests whereas W/O emulsion give negative results.
Staining test
Water soluble dyes such as methylene blue will uniformly dissolve in O/W emulsion on the other hand in W/O emulsion methylene blue will form clusters on the surface.
Filter paper test
A test paper soaked in cobalt chloride detects O/W emulsion by changing color from blue to pink.
Organoleptic parameters
O/W are not greasy on touch as opposed to W/O types that feel greasy to touch. Organoleptic parameters usually give an educated guess about the type / nature of the emulsions for surety analytical tests are recommended.
11.What are associated advantages and disadvantages of pharmaceutical emulsions?
| ADVANTAGES | DISADVANTAGES |
| Poorly water-soluble drugs can be delivered using oil base emulsions. | Emulsions are difficult to manufacture. |
| Emulsions mask unpleasant taste or odor. | Stability is affected by storage conditions |
| Prolonged release dosage forms can be formulated as emulsions. | Content uniformity is difficult to achieve. |
| Vitamins and other essential nutrients can be given to patient in the form of emulsions. | Susceptible to microbial degradation. |
| Medicines that rapidly undergo oxidation and hydrolysis are formulated as emulsion to enhance their stability. | Emulsions have short shelf life and phases separate eventually. |
| Can be used for diagnostic as well as treatment purposes. | Costly to manufacture. |
Conclusion
To summarize above blog emulsions are preparations that are manufactured by harmonizing two immiscible liquids, related to everyday industry including dairy, food, pharmaceuticals and cosmetics. Well folks if you need more information about the basics of emulsions you will have to completely read this blog and you can also check other useful articles on our website. Furthermore, if you want to develop your product contact our 24/7 available customer service. Happy reading!
Don't forget to share this post!
Vacuum Emulsifying Mixer Related Posts
Vacuum Emulsifying Mixer Related Products
Vacuum Emulsifying Mixer Related Videos
CONTACT US
Tell us your raw material and project budget to get quotations within 24 hours.
WhatsApp Us: +86 181 6426 8586

Want the best price & newest pharmaceutical machinery buying guide,tips and trends sent straightly to your box?Sign up for Aipak’s monthly newsletter,we’re free for your consultation and Offer you the most suitable solutions!
The Buyer's Guide
- Capsule Filling Buyer's Guide
- Blister Packaging Buyer's Guide
- Tablet Counting Buyer's Guide
- Tube Filling Buyer's Guide
- Cartoning Buyer's Guide
- Gummy Making Buyer's Guide
- CO2 Extraction Buyer's Guide
- Empty Capsules Buyer's Guide
- Suppository Filling Buyer's Guide
- Tablet Coating Buyer's Guide
- Tablet Press Buyer's Guide
- Softgel Encapsulation Buyer's Guide
Most Popular
- 7 Importance Of Pharmaceutical Packaging In Different Applications You Must Know
- 6 Advantages You Must Know About Tablet Counting Machine
- 8 Advantages of Blister Packaging You Must Know
- 6 Critical Applications of Automatic Capsule Filling Machine
- 6 Stations You must Know to Improve the Filling Quality of Automatic Capsule Filling Machine