Choosing the Right Equipment for Pilot Scale vs Full-Scale Production in Pharma
Scaling up the full-scale production in pharma is based on complex and multiple steps. That’s why pilot scale studies are conducted to evaluate the major effective stages, end-to-end expense, outcome, and the right equipment.
We are here aiming to help Pharmaceutical Manufacturers, Grow, and Thrive.
You can learn the expert skills to support your business and become more profitable and secure. This article on Choosing the Right Equipment for Pilot Scale vs Full-Scale Production in Pharma is particularly highlighting major machinery to begin your successful pharma journey.
1.Why is choosing the right equipment essential in pharmaceutical manufacturing?
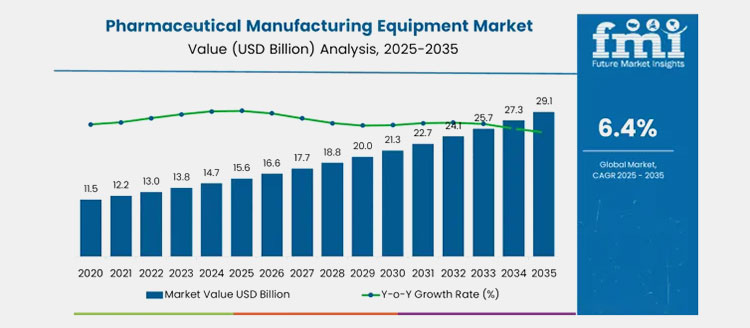
Pharmaceutical equipment market- Picture courtesy: Future Market
Choosing the right equipment is essential, that’s why pharmaceutical manufacturing market size to turn at CAGR of 6.4%. The current market rate is approx. USD 15.6 billion in 2025 which is to grow USD 29.1 billion by 2035. The equipment for pilot scale transformation to large scale drives the drug production on a large scale with consistency. The significant reasons for encouraging modernization shifts manufacturing processing to smart technology are described below:
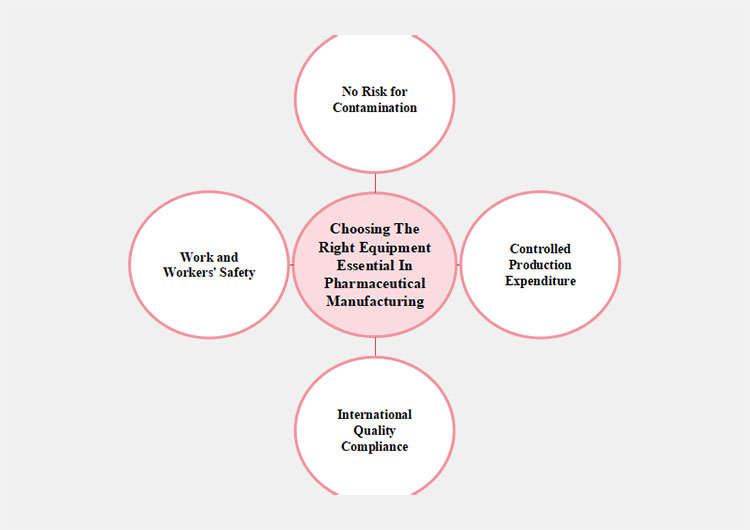
Reasons For Choosing the Right Equipment for Pharma Production
No Risk for Contamination
Choosing the ideal machines in pharma are commonly well designed and tightly enclosed. That means it doesn’t allow the entrance of external unwanted factors to interrupt the manufacturing process. This is a crucial factor that prevents contamination. Moreover, the machine material is easy to clean and avoids chances of cross-contamination in drug manufacturing, which ultimately keeps your medication process safe and full of quality.
Controlled Production Expenditure
The right equipment is the only option for a pharma manufacturing as it ensures that production meets controlled costs. For instance, it helps in lowering process downtime. This ultimately helps in boosting production as well as profitability.
International Quality Compliance
The right machine helps in meeting your product under Good Manufacturing Practices (GMPs). This is because the equipment design is stringent with compliance. This is also involved with validation and documentation. So, the formulation is safe for use and has ingredients and strength that manufacturers claim to have.
Work and Workers' Safety
The absolute and right equipment is ultimate solution for the safety of your workers and workflow. The pharma machine is designed with modern notifiers that alert you prior to any issues or hazards occurring. Also, these machines are designed to minimize dust exposure, heat liberation, and other risky parameters, hence, they’re safe for operators and the labs.
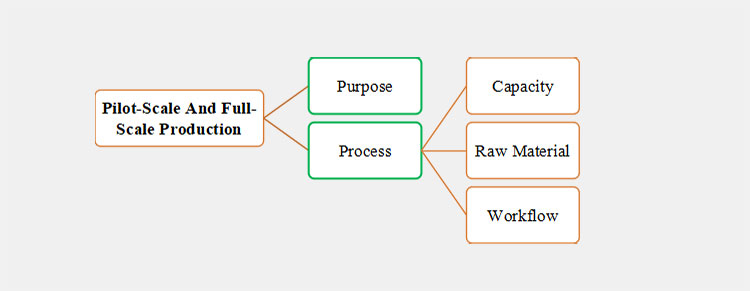
2.How do pilot-scale and full-scale production differ in purpose and process?
Pilot-Scale and Full-Scale Production
The major purpose and process difference between pilot scale and full-scale production are mentioned as follows:
Purpose and Process
Purpose
Purpose- Picture Courtesy: HyCON Lab
The pilot scale is the core study of production, including lab procedures and major commercial testing. The pilot test objectives are to assess, optimize, and produce basic literature related to study. Whereas the goal of full-scale is to produce a commercial product following a consistent batch-to-batch process and ready to deliver the formulation.
Process
Process- Picture Courtesy: Syntegon
The purpose and process of the pilot scale included a set of experimentation and data analysis, whereas the full scale followed major procedures and standard validation that provide a quality product that meets international regulatory standards.
Capacity
The pilot scale is a small study, and immediate modification in testing is flexible. The production capacity of such a study is based on short trials and is easy to manage. The large or full-scale production in pharma is indeed a continuous and large process with specialized systems with optimized formulation.
Raw Materials
The pilot scale testing is limited by the requirement of limited raw materials. Therefore, it is hooked with minimized costs. The full-scale production in pharma is based on a well-designed protocol; therefore, the materials are required in bulk with high investments.
Workforce
The workforce is based on a few members of R&D, quality control, and quality assurance, whose combined data can be approved for further production. The full-scale production is based on a huge team and experts to produce a reliable and quality product.
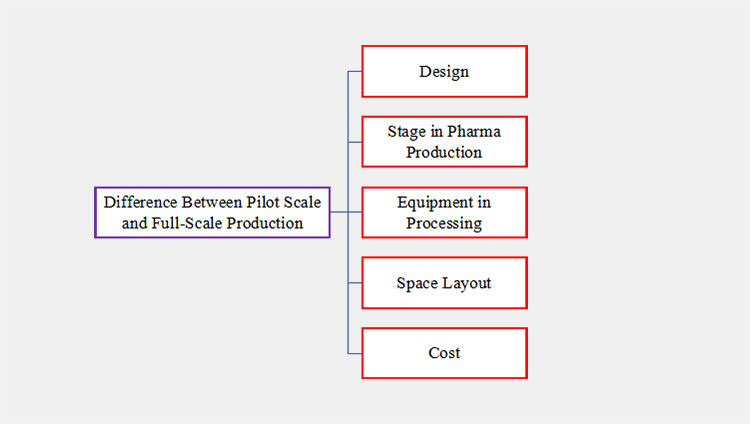
3.What Is the Difference Between Pilot Scale and Full-Scale Production?
The primary difference between pilot scale and full-scale production is described below:
Difference Between Pilot Scale and Full-Scale Production
| Design | Pilot-scale production means small-scale preparation associated with a small setup.
However, the full-scale production aims to commit to mass production, ensuring the process is reliable and can yield large market batches. |
| Stage in Pharma Production | Pilot-scale production is the primary stage to prepare a comprehensive record related to the product and develop the new formulation.
Full-scale production is a later stage that starts upon successful trials of the pilot study. Therefore, a consistent and large production supply is produced for market distribution. |
| Equipment in Processing | Due to limited raw materials and steps, the equipment in pilot-scale production is mostly compact and handy. They are easy to install, transfer, and run.
Whereas the equipment for processing the full-scale production in pharma are huge, requiring a large space for installation and meant to run large and continuous batches. |
| Space Layout | Modern R&D labs with innovative equipment are required to run pilot-scale production.
On the other hand, the large built-in production plants and a fully equipped lab with modern integrated machine space are required to run the large-scale operation. |
| Cost | The cost of a pilot scale is budget-friendly, and it helps in identifying the result with limited expenditure.
Full scale is associated with commercial production, therefore requiring a handsome investment to operate the process. |
4.What Factors Should You Consider When Selecting Equipment?
When purchasing the right equipment for pilot scale vs full scale production in pharma, you should consider the following points:
Adaptable & Multi-Purpose Equipment
Adaptable & Multi-Purpose Equipment
You should consider equipment with adaptable and multipurpose features that are suitable for running testing with modulated formulations. It should be flexible enough to deal with pilots as well as large-scale studies or production.
Machines with Data Recording
Machines with Data Recording
Whether pilot scale or large scale, the production equipment must be equipped with data monitoring and recording properties. Such features are helpful in interpreting, identifying, and optimizing the process or study; therefore, ideal for both pilot-scale and full-scale pharma production.
Easy Cleaning & Maintenance
Easy Cleaning & Maintenance- Picture Courtesy: Servico
Always prefer machines with robust and durable bodies. The right equipment for pilot scale vs full scale production in pharma must be easy to handle, easy to clean, and have hassle-free maintenance. This factor will be time and energy-saving for long-term processing.
Your Space Measurement
Laboratory Space Measurements- Picture Courtesy: Kewaunee
Before making the purchase, assess your designated space for equipment. In contrast to full-scale production, pilot production does not require a large space for equipment setup. Therefore, space measurement helps you to evaluate the area for effective adjustment of equipment. This will prevent congestion and workflow deviation.
Your Supplier Company
Pharmaceutical Equipment Supplier Companies
Whether pilot scale or full-scale production, choose a reliable supplier for equipment purchase. A trusted supplier offers excellent services, including equipment installation support, training, after-sales services, and compliance with regulatory requirements. Ensure the availability of spare parts, helping manuals, and proper documents. Don’t forget to conduct proper research via websites, reviews, etc., to reach a good company.
5.What Equipment Is Commonly Used at Each Scale?
| Pilot-Scale Equipment in Pharma | |
| Mixing & Blending | |
| Pilot-scale high-shear mixer
Lab Scale High Shear Mixer- Picture Courtesy: Silverson |
The machine is used for intense mixing and densification of the particles using the force of impellers, vacuum, and pressure. |
| Pilot V mixer / double cone blender
Pilot V mixer / double cone blender |
For mixing heat and moisture-sensitive materials, a small, benchtop, pilot V mixer or double cone blender is used, which allows tumbling and diffusive mixing. |
| Pilot bin blender
Lab scale bin blender |
An enclosed, dust-free, and flexible mixing occurs in a pilot bin blender, allowing easy detachable features. |
| Granulation | |
| Pilot Wet Granulator
Pilot Wet Granulator- Picture Courtesy: Allma.net |
Small-scale powder mixing with the inclusion of water and liquid binders mainly occurs in a wet granulator. |
| Drying | |
| Pilot Fluid Bed Dryer
Pilot Fluid Bed Dryer- Picture Courtesy: Sherwood |
A small and handy solution where hot air involves wet particle fluidization to eliminate moisture from powders and make them dry. |
| Milling & Sieving | |
| Pilot-Scale Oscillating Granulator
Pilot-scale oscillating granulator |
With the help of pilot-scale oscillating granulator, the particles break down by oscillating sharp blades and are made small and uniform. |
| Tablet / Capsule Production |
|
| Pilot Tablet Press
Pilot Tablet Press -Picture Courtesy: ERWEKA |
The machine combines and presses the powdery material into a tablet by using a compression force. This is mostly presented with manual and semi-automatic benchtop machines. |
| Pilot Capsule Filling Machine
Capsule Filling Machine- Pharma Test |
The series of empty capsules is filled and sealed either manually, semi-automatically, or automatically for testing batches. |
| Tablet Coater (Mini or Lab Coating Pan)
Pilot Tablet Coater |
The tablet coater helps with the uniform and flexible spraying of coating layers to stabilize the pilot tablet’s appearance. |
| Liquid & Semi-Solid Preparation | |
| Pilot Homogenizer/Emulsifier
Pilot Homogenizer/Emulsifier- Picture Courtesy: DMC
|
To manufacture stable liquid and semi-liquid formulations, the homogenizer or emulsifier is used, which gives an emulsified solution by the end of the process. |
| Pilot Syrup or Suspension Preparation Tank
Pilot Syrup or Suspension Preparation Tank |
The pharma prepared liquid syrup or suspension are mostly stored in storage tanks. These are facilitated stirrer and insulated jackets to maintain temperature, homogeneity, and avoid sedimentation. |
| Pilot ointment/cream mixer
Pilot Ointment/Cream Mixer-Picture Courtesy: Cream Manufacturing Equipment |
To blend the water phase in the oil phase or vice versa, the ointment/cream mixer is used to prepare testing formulations. |
| Sterile & Aseptic Processing | |
| Pilot BFS Machine
Pilot BFS Machine |
For hygienic and error-free filling of formulation, the pilot BFS or blow-fill-seal machine is used. This machines helps in sterile filling of preparation with hermetically sealing under a controlled and aseptic working principles. |
| Pilot Aseptic Filling and Sealing Unit
Pilot Aseptic Filling and Sealing Unit- Picture Courtesy: ALFA |
The sterilized containers are filled and sealed in an aseptic filling and sealing unit. It allows users’ and medicine’s safety. This is designed in a way ensuring positive pressure to keep away unwanted contaminants from the lab’s environment |
| Packaging | |
| Pilot Blister Packaging Machine
Pilot Blister Packaging Machine |
The medication is individually packed in blister cavities to make it available for testing trials safely. |
| Pilot Bottle Filling and Capping Line
Pilot Bottle Filling and Capping Line- Picture Courtesy: NEOSTARPACK |
The bottle filling and capping machine is used to fill materials to be dispensed accurately in small testing bottles with simultaneous capping in an organized manner. |
| Pilot Sachet or Stick Pack Filler
Pilot Sachet or Stick Pack Filler |
The semi-automatic machines are mainly used for forming, filling, and sealing of sachets and stick packs, allowing consistent production of clinical batches. |
Case Story for Pilot Production
The Catalent Pharma Solutions were subjected to a switch from mid-size pharma production of oral tablets (ER) from lab to pilot scale batches. This is involved with scaling up formulation from 1kg to 30 kg prior to moving to the clinical trial phase III. The objective was to formulate a consistent tablet batch while using a modified polymer matrix.
Challenges
The challenges of production are described as follows:
Inconsistency in dissolution profile due to polymer coating variation.
Presence of moisture content due to ineffective drying.
Shortage of time for clinical trials testing.
Addressing the Challenges for Pilot-Scale
Installed pilot-scale equipment such as a high shear mixer and a pilot fluid bed dryer for wet granulation.
Implemented GMP-compliant clinical trial testings to get the target results within the specified protocol.
Scheduled DoE for various significant parameters, such as identifying mixing time, binding thickness, and temperature.
Outcomes
By following the implementation steps, the target dissolution and hardness of coated tablets were achieved.
The prepared data, validation test, and protocols were successfully transitioned to the commercial production setup.
| Full-Scale Equipment in Pharma | |
| Large-Capacity V Mixer/Bin Blender
AIPAK V Mixer |
These are the large machines that allow tumbling of dry powders in a V-shaped tank. This is an efficient solution for managing bulk materials. |
| Granulation
|
|
| Industrial High-Shear Mixer Granulator
AIPAK High Shear Mixer Granulator |
The machine is used for bulk treatment of powders to make granules with the support of intense impellers and choppers.
The combined action allows consistent mixing properties and uniform granule formation that is used for medicines such as capsules, tablets, and related pharma products. |
| Drying | |
| Industrial Fluid Bed Dryer
AIPAK Fluid Bed Dryer |
This equipment is used to dry the materials (powders, granules, etc.).
With the help of hot air that suspends the particles in the air and eliminates the presence of water. The machine is ideal for uniform drying of the particles without any overheating. As compared to the fluidized bed granulator, this machine is only efficient for drying, but not mixing. |
| Tablet / Capsule Production | |
| High-Speed Rotary Tablet Press
AIPAK Tablet Press |
The high-speed rotary tablet press combines and compresses the mixed ingredients into a tablet with the help of punches and dies. The machine is available in semi-automatic and automatic high-speed rotary tablet presses to deal with bulk production batches in pharma. |
| Automatic Capsule Filling Machine
AIPAK Automatic Capsule Filling Machine |
The automatic capsule filling machine is used to dispense pharmaceutical powders, granules, micro-tablets, and pellets into the empty capsule shells.
The machine is capable of loading, opening, filling, and locking of capsule body automatically. The quality solution that allows capsules to fill in at a high speed without inaccuracies and is in demand not just for production in pharma but other related industries. |
| Tablet Coating Machine
AIPAK Tablet Coating Machine |
The machine allows spraying and drying of the coating materials over tablets, granules, or pellet bed uniformly.
The material bed is circulating in a rotary drum, and the machine is featured with a film spray that allows controlled airflow to form an even layer of coating film in a large-scale production batch. |
| Liquid & Semi-Solid Preparation | |
| Syrup Filling Line
AIPAK Engineering Syrup Filling Line |
An industrial syrup filling line is an integrated solution for syrup filling, which is based on a set of machines such as a bottle unscrambler, a syrup filling machine, a capping machine, an induction sealing machine, and a labeling machine to produce a large-scale production in pharma. |
| Vial Filling and Sealing Line
AIPAK Engineering Vial Filling and Sealing Line |
A large and sterile solution for feeding, washing, dehydrogenation, filling, and capping of the vials with leakproof packaging.
The main preparations are included with liquids, powders and placed into the vials with sturdy rubber stoppers. To make them safe from the external environment. |
| Ampoule Filling Machine
AIPAK Engineering Ampoule Filling Machine |
An ampoule filling machine precisely fills certain proportions of sterile liquid formulations into ampoules and hermetically seals each ampoule.
The sealing is mainly achieved with heat that melts the glass layers and forms a fixed sealing upon cooling. |
| Vacuum Emulsifying Mixer
AIPAK vacuum emulsifying system |
The vacuum emulsifying mixer facilitates fast and quality mixing, dispersing of viscous materials to form a stable product. The working principle of the machine is based on intense impellers and a vacuum to produce a formulation with even distribution of particles without risk for sedimentation.
This machine is ideal for dealing with creams, lotions, gels, syrups, emulsions, etc. |
| Tube Filling Machine
AIPAK Tube Filling Machine |
A tube filling machine is an automatic, high-tech pharma equipment that precisely fills the formulation in an individual tube.
The tube feeding, filling, thermal sealing, trimming, and processing are automatically adopted with a smart high-sensor control system. The machine is specially designed for viscous formulations, such as pharmaceutical creams, ointments, and many other products. |
| Sterile & Parenteral Processing | |
| Full-Scale BFS (Blow-Fill-Seal) Machine
AIPAK Engineering Full-Scale BFS (Blow-Fill-Seal) Machine |
A full-scale blow fill seal or BFS machine is used for sterile packaging of semi-liquid and liquid medicinal preparations in large-scale production in pharma. The setup ensures sterile and controlled procedures with no risk for contamination. |
| Sterile Filtration and Autoclave System
Sterile Filtration and Autoclave System |
To block the entrance of microorganisms and foreign particles, the filtration system is used, which removes the presence of unwanted materials in medicines.
However, the autoclave system is designed to kill microorganisms once they are subjected to high pressure and temperature of 121℃, which denatures them. |
| Packaging & End-of-Line | |
| Blister packaging line
AIPAK Blister packaging line |
A blister packaging line is a complete automatic packing solution to create and pack blister packs into boxes.
The commonly used setup for full-scale production in pharma is where a series of products are positioned into each blister cavity, followed by lidding, and the process is once they’re placed into retail cartons within a limited time. |
| Bottle unscrambling, filling & labeling line
|
The typical production line that is presented with integration of a bottle unscrambling machine for orientation of random bottles, filling machines, and an automatic labeling machine for applications of labels over the bottles.
The purpose of the setup is to improve the fast working of full-scale production in pharma with more accurate and professional results. |
| Cartoning Machine
AIPAK Cartoning Machine |
A cartoning machine that folds, side seams, and seals the edges of paperboard or cardboard into a carton.
The machine identifies and places the items with auto-sensors and into cartons and closes the boxes automatically to ensure qualified packing. |
| Case packer & pelletizer
Case Packer and Pelletizer- Picture Courtesy: Paxicom Group |
For full-scale production in pharma, a case packer and pelletizer are used for final packaging before market distribution.
Machines help in creating case packing, and sets of packed cartons are stacked into the pellets automatically, which allows flexible transportation with reduced manual labor. |
Case Story for Large-Scale Production
The Cambrex (global contract development and manufacturing sector) was approached by a European pharma company for scaling up an API on a commercial scale. The APIs were high-potential molecules with occupational exposure of only 1–10 µg/m³.
Challenge
The challenges of the study are described as follows:
The demand was to upgrade level in 10thkilograms annually, which is significant.
The multiple departments were to be involved in several analysis studies safely.
Addressing Challenges for Large-Scale Production
The validation and build-in of the new manufacturing area took place under cGMP facilities to meet regulatory requirements for dealing with high-potential API.
The training of workers, SOPs re-validation, and OQ, PQ, IQ, and associated qualifications documentation of equipment are followed and updated.
The company’s collaborative timeline and quality requirements are guaranteed with time-to-time investigation.
Outcomes
As a result of successful work implementation, Cambrex became a strong partner to provide a large-scale API production.
The scaling up was done within the time limit safely that showing strong evidence of expertise, experience, and potential authoritativeness.
6.What types of mixers, granulators, or dryers are used for small-batch testing?
For small-batch testing, the types of mixers, granulators, or dryers are commonly small or lab-scale units. They are also known as benchtop equipment and are used for pilot testing. For example:
Double Planetary Mixer
Double Planetary Mixer- Picture Courtesy: Ele-mix
A machine designed with rotary blades looks like a planetary system allows rapid and uniform mixing. The mixer is used for wet granulation and is ideal for lab or small batch testing.
Benchtop Double Cone Blender
Benchtop Double Cone Blender
For small-scale blending and mixing powders and granules, the double cone blender is also significantly applied to get uniform mixing and homogeneous materials.
Mini Rapid Mixer Granulator
Tabletop Mini Rapid Mixer Granulator
This is designed for small-scale lab testing. The fast impeller allows fast and super easy granulation, which is similar as subjected to large machines.
Vacuum and Freeze Dryer
Vacuum and Freeze dryers- Picture Courtesy: Faithful
Vacuum and freeze-dryers are commonly used machines in small lab testing. The vacuum dryer, where material is dried under reduced temperature and pressure, is considered ideal for heat-sensitive products. In case of freezing, the sublimation process is used to remove water content.
7.What large-scale systems are typically used for mass production?
A large system for full-scale pharma production is mainly automatic machines that can deal with mass or high capacities. They are categorized into main branches such as:
Solid Dosage Formulation
Solid dosage formulation, large-scale system
The typically used systems are involved with mixers, granulators, presses, filling, coating, and packaging lines. The packaging lines include counting lines, blister packaging lines, and others.
Liquid And Semi-Solid Dosage Formulation
Liquid and semi-solid dosage Formulation- Picture Courtesy: Pharmec
The liquid large-scale systems are typically involved with mixing machines like emulsifying mixers (viscous formulation), filtration machines, filling machines, washing machines, and capping. Whereas the packaging lines, such as syrup filling lines, bottle filling lines that are composed of a complete set of equipment required for mass production.
Parenteral Formulations
AIPAK Engineering Aseptic Liquid Vial Production Line
The parenteral or sterile formulation mainly uses systems included with a large filling and sealing line. For example, BFS, vials, ampoules, IV infusions, and related machines. Also, filtration, autoclave units, cleaning machines (CIP, SIP systems), water sterilization plants, etc., are known as the significant units of parenteral formulations in mass production.
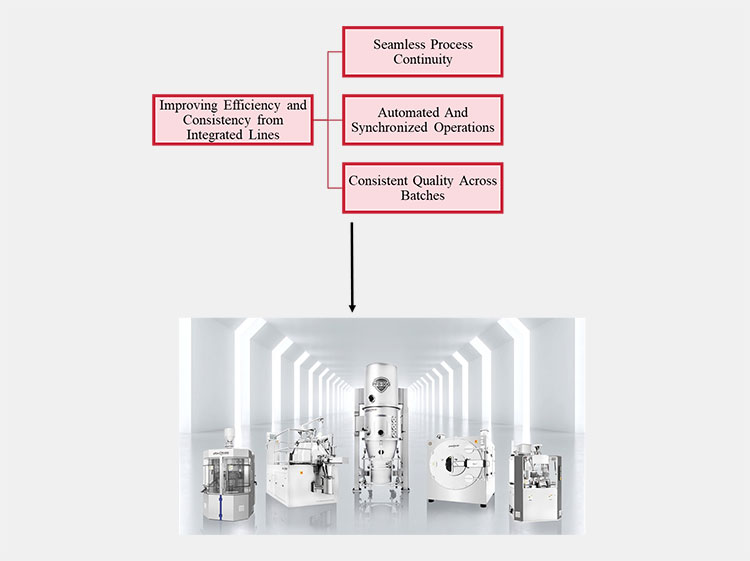
8.How do integrated lines improve efficiency and product consistency?
The integrated lines are commonly required in full-scale production in pharma for bulk management of batches. The prime reasons for their need to improve efficiency and product consistency are discussed below:
Integrated Lines Improving Efficiency and Product Consistency
Seamless Process Continuity
Setting up integration between pilot scale and full-scale equipment enables seamless continuity in the production process, avoiding any potential risk of limitations and process deviations in critical parameters such as mixing duration, temperature control, and drying rate.
Automated And Synchronized Operations
Integrated production lines connect all the equipment under an identified single control system, directly minimizing manual interference in a large production. During work, the common challenge is to retrieve past methods or protocols quickly. The automatically synchronized properties deliver an easy solution with just a single click. This mechanism ensures an efficient and speedy production process while reducing the risk of errors and providing previous method records.
Consistent Quality Across Batches
The integrated lines help in attaining uniform quality across all batches. Real-time monitoring of critical parameters such as moisture level, particle size, or blending consistency leads to stable quality in all batches.
9.What Challenges Occur When Scaling Up from Pilot to Full-Scale?
The scaling up from pilot scale to full-scale production isn’t just about making larger batches- it’s far from a linear process. In the section below, you will explore significant challenges that come up when transitioning from pilot to full-scale in the pharmaceutical industry.
Challenge: Measurement /Scale Difference
Measurement /Scale Difference- Picture Courtesy: Ilovephd
Volumes of batches are often just a few litres or kilograms at a small scale, making it easy to visually examine and physically sample the product. Whereas batch volumes can reach up to thousands of litres or a few tons. Drawing representative samples from such a huge vessel is difficult, limiting which manual or visual assessment.
Solution: To address the challenge, full-scale production should integrate in-line sensors and automated probes to continuously measure process parameters across the entire instrument.
Challenge: Process Optimization and Reproducibility
Process Optimization and Reproducibility- Picture Courtesy: Aborpharmchem
At the pilot scale, processes are very easy to monitor, modify, and even reproduce because of small batch sizes. In contrast, full-scale manufacturing encounters greater variations of temperature, pressure, moisture level, and feed rate, drastically impacting batch reproducibility and process efficiency.
Solution: To address the challenge, an advanced process control system integrated with real-time monitoring and data analytics should be installed for superior reproducibility.
Challenge: Non-Uniform Heat Transfer
Non-Uniform Heat Transfer- Picture Courtesy: Nature
As compared to pilot scale, larger vessels at a commercial scale generate more heat than small vessels, while heat-transfer capacity jackets or coils increase at a slower rate. Consequently, heat removal or distribution is less uniform in full-scale devices, increasing the risk of thermal instability and runaway reactions. This may jeopardize operator safety, equipment integrity, and product quality.
Solution: To solve the challenge an efficient cooling systems and advanced temperature control sensors are integrated. It helps in large systems to uniformly control heat transfer in full-scale process flow.
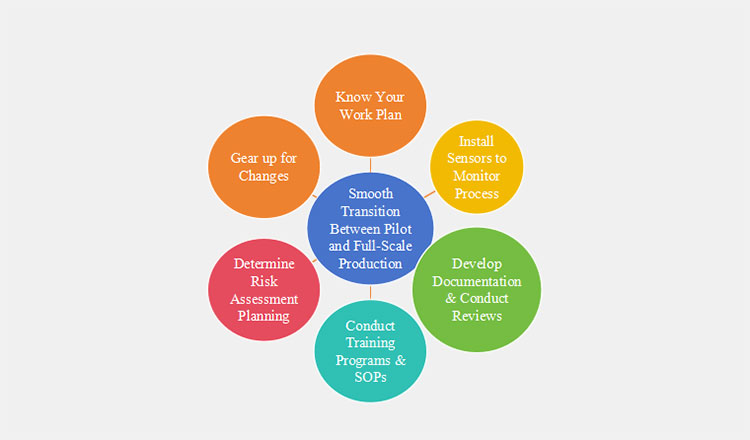
10.How Can You Ensure a Smooth Transition Between Pilot and Full-Scale Production?
Steps for Smooth Transition Between Pilot and Full-Scale Production
To ensure a smooth shift from pilot production to commercial scale production, you must consider the following factors.
Know Your Work Plan
For a robust transition, it is mandatory to have a concise work plan that includes production process steps, equipment requirements, and critical parameters analysis. This will ensure everyone understands the agenda before moving to large-scale production.
Install Sensors to Monitor Process
Installing sensors helps in making you connected with the whole process. The commonly used sensors are included as SCADA (Supervisory Control and Data Acquisition), PAT (Process Analytical Technology), DCS (Distributed Control System), etc. It ensures the real-time, continuous tracking of process steps critically.
For example, temperature, humidity, speed, particle sizes, etc. Additionally, automatic control over all integrated machines enables hassle-free process and smooth transition from pilot to large-scale production.
Develop Documentation & Conduct Reviews
Proper documentation during pilot production ensures a precise shift to large-scale production. Develop accurate documentation of batch data, critical parameters, and equipment control settings. Continuous reviews during pilot production validate large-scale production expectations and pinpoint required adjustments before transition.
Conduct Training Programs & SOPs
Effective training and proper SOPs consistently aid the production process. The team must have clear knowledge of equipment requirements, production process, and safety standards. Meanwhile, concise SOPs provide instructions, ensuring seamless operation.
Determine Risk Assessment Planning
Risk assessment planning is carried out in order to identify any potential risks to ensure high commercial production. Strategic risk assessment helps avoid process deviations and failures, enabling the team to plan for limitations and to prepare safety measures to overcome them.
Gear up for Changes
The transition pilot and full-scale production meet many hurdles related to documentation, equipment, planning, etc. Therefore, the pharma companies must be ready for challenges. This involves approval or rejection of many programs, projects, rules, etc., that can affect your budget, timelines, and result in more expenses.
Conclusion
In the pharmaceutical arena, choosing the right equipment for pilot scale VS full scale production in pharma will offer the best goals related to developing medications. Additionally, the right knowledge plays an advancing role in processing the broad range and quality of solutions. If you’re looking for the right equipment in the field of pharma production. AIPAK’s team is right there to extend the contribution related to machines and support the process. To make your problems easier, just message us to start fast communication to boost your business.
Don't forget to share this post!
Blister Packaging Machine Related Posts
Blister Packaging Machine Related Products
Blister Packaging Machine Related Videos
CONTACT US
Tell us your raw material and project budget to get quotations within 24 hours.
WhatsApp Us: +86 181 6426 8586

Want the best price & newest pharmaceutical machinery buying guide,tips and trends sent straightly to your box?Sign up for Aipak’s monthly newsletter,we’re free for your consultation and Offer you the most suitable solutions!
The Buyer's Guide
- Capsule Filling Buyer's Guide
- Blister Packaging Buyer's Guide
- Tablet Counting Buyer's Guide
- Tube Filling Buyer's Guide
- Cartoning Buyer's Guide
- Gummy Making Buyer's Guide
- CO2 Extraction Buyer's Guide
- Empty Capsules Buyer's Guide
- Suppository Filling Buyer's Guide
- Tablet Coating Buyer's Guide
- Tablet Press Buyer's Guide
- Softgel Encapsulation Buyer's Guide
Most Popular
- 7 Importance Of Pharmaceutical Packaging In Different Applications You Must Know
- 6 Advantages You Must Know About Tablet Counting Machine
- 8 Advantages of Blister Packaging You Must Know
- 6 Critical Applications of Automatic Capsule Filling Machine
- 6 Stations You must Know to Improve the Filling Quality of Automatic Capsule Filling Machine