GMP, CE, FDA Certification- Why They Matter For Pharmaceutical Packaging Equipment
Pharmaceutical companies cares about the safety and health of the patients. From formulation to the final packaging of each product, every step required precision. To ensure that each step is carried out effectively in a machine, they should meet the international quality and safety standards. But how will you know they meets the mentioned requirement? The answer lies in GMP, CE, and FDA certification. These certifications are not just an approval but it ensures the safely operation of the machine. It also develop the trust between manufacturer, regulators and consumers.
This article will guide you why they matter for pharmaceutical packaging equipment? What are these? Why are they important? So, let’s uncover the magic lies in these certifications!
1.What does GMP (Good Manufacturing Practice) ensure in equipment production?
GMP ensures equipment production-Picture courtesy:oxmaint.com
GMP means the quality assurance of the products to be manufactured, installed, operated and maintenance. In this practices, everything about the equipment has been carefully handled- from material selection for the production of machinery, its design, the installation, the training of the operator, to the maintenance of the machine. Consistent batch production is necessary so, for that each step should be documented to avoid any hindrance during its operation.
2.What does CE certification represent for safety and reliability in the EU market?
CE certification in Europe-Picture courtesy: mirabake.com
The CE certification indicates that the machinery has been tested and evaluated that meets the EU safety, manufacturing and environmental protection. You can manufactured anywhere in the world and can transport the products without any other proof if you have CE certification. Without this certification, you can’t place and sell the equipment’s in the EU, EEA countries and Turkey. Because this certification indicates the safety, health and environmental protection.
This certificate also represent the reliability, the product having this tag indicate that regulatory requirements have been considered and compliant with EU rules.
3.Why is FDA approval essential for pharmaceutical packaging machinery in the U.S.?
FDA approval in U.S.-Picture courtesy: arrotek.com
Food and drug administration (FDA) approval is essential for pharmaceutical packaging machinery in the U.S. as it ensures the safety, efficacy and stability of the pharmaceutical products. This approval verifies that the packaging material should be non-reactive with the formulated drugs. The material used for machinery should be safe. The packaging style should be intact. The ingredients labels and traceability must be clear.
Within the U.S, the FDA approval is compulsory as this approval enforce American laws for public health protection.
4.What does it mean when a manufacturer like AIPAK Pharma claims to be “GMP, CE, FDA compliant”?
AIPAK Machinery Certificate
When a manufacturer like AIPAK Pharma claims to be “GMP, CE, FDA” complaint then this means that the machines are safe to use, materials used in its construction are safe for the drug ingredients, consistent products production, accurate labelling and traceability. You can sell and place the machines globally in multiple regions including Europe and U.S.
5.Why are GMP, CE, and FDA certification so important in the pharmaceutical equipment industry?
Softgel encapsulation production line certificate-Picture courtesy: Aipak
The GMP, CE, and FDA certifications play a vital role in the pharmaceutical equipment industry. GMP certificate ensures you that the products are produced and monitored consistently and they always meet the established quality standards. This guarantee you the products are free of defects and contamination.
As the pharmaceutical equipments should be responsible for safe products manufacturing so, CE marking shows the product complies with European Union’s standards for health, safety and environmental protections. It ensures that the product has been assessed through careful design checks, risk assessment and when necessary, they gone through clinical evaluation to prove that the products are safe to use.
FDA certificate is very important in pharmaceutical equipments industry. In this, the products are passed through the world most thorough review’s processes. This includes the lab testing, inspection of labeled products which ensures the safety and effectiveness of the products.
6.What are GMP, CE, and FDA certification?
GMP-Picture courtesy: medellasoftgel.com
FDA and CE signs-Picture courtesy: defibsolutions.eu
GMP stands for Good Manufacturing Processes. This involves the raw material handling, training of employees and hygienic workplace, processes and documentation of machinery.
CE stands for “Conformité Européenne”. If the equipment has CE mark then this means the product meets the safety and performance standards set by European Union.
FDA stands for Food and Drug Administration. It means that the American government has declared the safe medicine and other products can be used, imported and sold in the United States.
7.Why do these certification matter for pharmaceutical packaging equipment?
Importance of certification in pharmaceutical packaging equipment-Picture courtesy: reeltech.sk
The Certificates such as GMP, CE, and FDA are not just a marks but they guarantee you the safety, quality and reliability of the pharmaceutical packaging equipment’s. In industries especially pharmaceutical industry, regulatory compliances is non-negotiable. Also, these certificates develop a trust of the consumers. That’s why, these ensures you the operational excellence and market compliance.
8.How do AIPAK’s features like “CIP/SIP systems” and “sterile manufacturing design” meet these global standards?
CIP/SIP systems-Picture courtesy: aipakengineering.com
AIPAK features like CIP (clean-in-place), SIP (sterilize-in-place) systems and sterile manufacturing design to meet the global standards like GMP, CE and FDA. Here is how!
The GMP standard meets:
CIP/ SIP systems:
GMP standard requires the machine must be clean and prevent cross contamination. The CIP system ensures the machine parts are clean with the help of internal pipes without dissemblance of the whole machine parts. This guarantee the safety without cross contamination. The SIP is the extended form of CIP in which the steam is passed through the machine parts which ensures the sanity.
Sterile manufacturing design:
According to GMP, the surface must be smooth so that, it can easily be clean and non-reactive with the drug ingredients. The machinery offered by AIPAK is made up of stainless steel which can easily be clean and buildup of microbes are prevented.
The CE standard meets:
CIP/SIP systems:
The CE ensures the machinery should operate hygienically and safely. The CIP/SIP systems offers the least exposure of human with the machine ensuring the machine environment should maintained hygienically.
Sterile manufacturing design:
AIPAK machinery designs are enclosed which ensures the safety of the operator during the preparation of ingredients and packaging of drugs so, this sterile manufacturing design meets the CE standards.
The FDA standard:
CIP/SIP systems of the machines:
According to FDA, the machine must be cleaned and maintained. AIPAK’s CIP/ SIP systems ensures validation, documentation and repeatability which ensures the safety and maintenance of the machine.
Sterile manufacturing design of the machine:
The AIPAK equipments are closed-system designs and uniform air-flow ensures product sterility and prevents the products degradation which meets the FDA standard.
9.How do GMP, CE, and FDA certifications enhance a manufacturer’s international reputation and export potential?
Certifications enhance international reputation and export potential-Picture courtesy: plutuseducation.com
GMP, CE and FDA certifications enhance a manufacturer’s international reputation and export potential by the following ways:
Credibility and trust with buyers:
The GMP certification ensures the safety and quality of the product. This enhances the trust between the manufacturer’s, customers and distributors.
The CE mark guarantee the safety and consistent productivity of the machine. This means that the product complies with European safety and health. You can export the machine other than Europe as well due to international recognition.
The FDA benchmark adds one of the most regulatory frameworks, enhances the international reputation.
Competitive differentiation and brand strengthening:
The brand claiming the GMP certification means it is competitive, as the GMP shows the product safety and quality. If a brand providing you GMP,CE and FDA certifications, it means it is commitment to the product lifespan and quality. In this way, the brand can be strengthened.
Future proofing and global alignment of standard:
The global trust can be developed by each other reviewing. For example, you can export CE certified machines to other countries as well other than the Europe. So, these certifications develop a confidence among manufacturer to position themselves in the future markets and regulatory changes rather than sticking to one region.
10.How should “GMP/CE/FDA Compliant” claims be presented on websites or product pages?
Certificate on website page-Picture courtesy: AIPAK
Claims such as GMP, CE, and FDA complaints should be presented on websites or product pages clearly and accurately. The presentation should be as follows:
If the product is manufactured in GMP compliant facility, then you should write as “the product is made according to GMP standard”.
You should not use statements that suggest universal approval by the authorities. If the product is GMP, CE and ISO certified, then you should claim as “we offer GMP, CE and ISO certified manufacturing standards”.
Always display compliance information in appropriate place in the “About Us” section.
If certain machine models are certified, then clearly mention the model.
11.How do companies like AIPAK Pharma maintain certification through continuous improvement and automated quality control?
Continuous improvement-Picture courtesy: flaticon.com
Companies like AIPAK Pharma maintain certifications through continuous improvement and automated quality control. They monitored the quality and improvement in every step of production. The gaps are to be noted before affecting the quality of the product. Continuous training to the operators by keeping knowledge up-to-dates is the part of improvement. This continuous improvement shows that the company compliant over time even regulations evolve. The AIPAK equipments have automated systems that record data for every batch, which can be documented for GMP and FDA audits. This ensures the products meet the global trust and the company should internationally be recognized and protecting its reputation.
12.How do certified machines improve production reliability and quality?
Certificate of machinery-Picture courtesy: icapsulepack.com
The certified machines are commitment to the production reliability and quality. These machines are run through testing and evaluating procedure before approval to use. Everything related to machine, from the material used in its construction, design to the products production, everything has to be evaluated. It ensures you the smooth production of the products without interruption and exposure to the contamination.
For companies and large businesses, this reliability assure you the consistent batch production. You can get a large batches of the product smoothly.
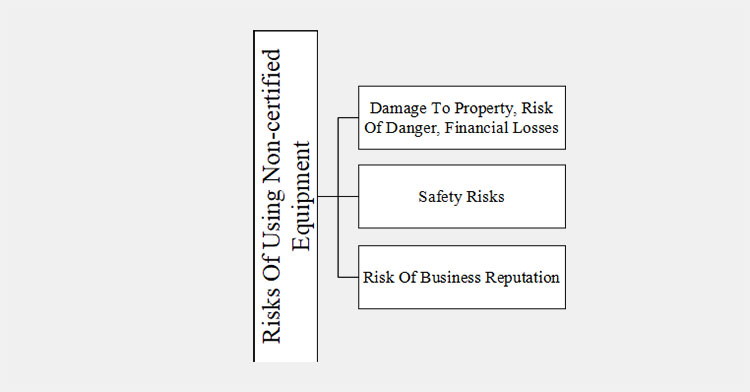
13.What are the risks of using non-certified equipment?
The use of un-certified equipments are risky for the consumers as well as to the businesses. These machines may lead to damage to the property, risk of danger and financial losses. The products may also be contaminated, inconsistent dosage and improper packaging. The reason behind these are, as they are not undergo through thorough testing and evaluation.
For users, the un-certified machines can lead to serious safety risks. The electrical products can cause electric shock, malfunction and breakdown.
The businesses reputation can be effected because of the poor functioning of the machine and inconsistent batch production.
14.How can manufacturers obtain or verify equipment certifications?
Verification of equipment-Picture courtesy: pilz.com
Manufacturers can obtain or verify equipment certifications by following the national and international regulatory authorities and certification bodies. The following steps should be followed:
The equipments manufactured should be provide to the authorities for testing.
The manufacturer should provide all the necessary documents such as documents of risk assessment and risk reduction, photographs of the machine, detailed technical specifications etc.
The equipments undergo through a process of testing such as safety, performance and compliance.
Once all the requirements meet the standards, the authority issue the certifications such as GMP, CE and FDA.
To verify the certifications, the buyers should check the validation and should keep on renewal them to keep their certification valid.
15.How are global harmonization efforts (ISO, WHO, EU MDR) affecting certification requirements?
International organizations-Picture courtesy: laserconn.com
Global harmonization means that the international organizations such as ISO, WHO, EU MDR are working together to make medical devices and pharmaceutical certification rules most consistent in every corner of the world. They are raising the bar for clinical evidences, and creating a route that reduce duplicate audit across the markets.
Strict quality and safety standards:
Manufacturers are expected to follow the quality management systems (QMS) based on international standards such as ISO 13485. This simplifies global compliance planning and ensures that the products are effective, safe to use and produced according to good manufacturing practices.
Clinical evidence expectations:
According to EU MDR (European Medical Device Regulation) and other similar global rules, the manufacturer should provide the clinical evidence throughout the lifecycle of the equipment. The regulators should provide a real-life data and clinical evidences not just once, or before the approval but should provide the data even the equipments are on the market.
Unified documentations:
The manufacturers should provide the risk management files, the detailed technical documentations which should be aligned with standards like ISO. This demonstrate the device can operate safely.
New/ expanded reliance and prequalification pathways( WHO & IMDRF):
The world health organization (WHO) prequalification programs and international medical devices regulators forums (IMDRF) convergence documents have created a new programs that reduce duplicate audits. This can create the trust and use each other product reviews and reuse evidence for multiple markets.
Conclusion:
It is concluded that the international certifications such as GMP, CE, and FDA are non-negotiable, as they ensure smooth machine operation, product hygiene, and consistent dosage. These are the key factors for maintaining business reputation and consumer trust. These certifications verify that products, especially pharmaceuticals containing APIs, meet strict quality and safety standards through proper testing and evaluation, guaranteeing consistent batch production. In contrast, using non-certified machines can lead to serious risks and quality issues, making these certifications essential for any reliable manufacturer.
Don't forget to share this post!
Blister Packaging Machine Related Posts
Blister Packaging Machine Related Products
Blister Packaging Machine Related Videos
CONTACT US
Tell us your raw material and project budget to get quotations within 24 hours.
WhatsApp Us: +86 181 6426 8586
Want the best price & newest pharmaceutical machinery buying guide,tips and trends sent straightly to your box?Sign up for Aipak’s monthly newsletter,we’re free for your consultation and Offer you the most suitable solutions!
The Buyer's Guide
- Capsule Filling Buyer's Guide
- Blister Packaging Buyer's Guide
- Tablet Counting Buyer's Guide
- Tube Filling Buyer's Guide
- Cartoning Buyer's Guide
- Gummy Making Buyer's Guide
- CO2 Extraction Buyer's Guide
- Empty Capsules Buyer's Guide
- Suppository Filling Buyer's Guide
- Tablet Coating Buyer's Guide
- Tablet Press Buyer's Guide
- Softgel Encapsulation Buyer's Guide
Most Popular
- 7 Importance Of Pharmaceutical Packaging In Different Applications You Must Know
- 6 Advantages You Must Know About Tablet Counting Machine
- 8 Advantages of Blister Packaging You Must Know
- 6 Critical Applications of Automatic Capsule Filling Machine
- 6 Stations You must Know to Improve the Filling Quality of Automatic Capsule Filling Machine