How To Control Compressed Air Quality In Pharmaceutical Process?
Air through the air compressor to do mechanical work to reduce the volume, the pressure is called compressed air. Compressed air has the characteristics of clarity and transparency, convenient transportation, no condensation, and can work in a variety of adverse environments.
This excellent application performance makes it widely used in pharmaceutical, video, light industry, biochemical and other manufacturing industries.
In the pharmaceutical industry, compressed air can be directly in contact with materials and drugs when used as process source gas.
While in the use as power source, although not direct contact with drugs, but most are used in the clean area. Therefore, compressed air for pharmaceutical use must be clean compressed air.
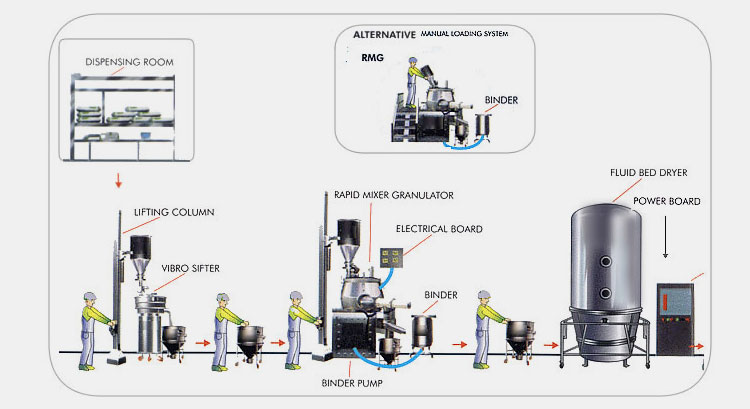
In pharmaceutical factories, compressed air is mainly used for filling machines in liquid preparations, granulator machines, pulping machines, capsule filling machines, tablet packing machines, printing machines, extraction tanks in the extraction process in solid preparations.
The quality of compressed air in pharmaceutical factories is mainly to control its water content, oil content, dust content and biological particle content, but also require compressed air odorless.
Compressed air containing oil can contaminate the drug if it comes into direct contact with it. Compressed air containing liquid water droplets will cause corrosion of pipes, valves and equipment, and rust stains from water droplets will also contaminate drugs, affecting the quality of drugs.
About the quality of compressed air, some experts believe that 4 indicators can be used to measure:
First, water content is measured by pressure dew point.
Second, dust quantity is expressed by dust particle size and quantity per unit volume.
Third, oil content is expressed as oil mass per unit volume.
Fourth, biological indicators were expressed as the number of microorganisms per unit volume and bacterial endotoxin.
In addition, the quality indicators of compressed air for pharmaceutical use can be determined according to the following principles:
The general principle is that the quality of compressed air used in the clean area cannot pollute the cleanliness of the clean area. Compressed air in direct contact with materials and drugs (including intermediate products and inner packaging materials) shall not affect the quality and stability of materials and drugs.
In other words, the compressed air quality index applied to the clean area should not be lower than the cleanliness level requirement of the clean area.
At present, many pharmaceutical companies are using clean compressed air system, which refers to a system composed of equipment to generate, process and store compressed air. The system refers to a system consisting of equipment for generating, handling and storing compressed air. Functionally, it can be divided into three parts:
First, produce compressed air part, including air compressor and its control system, cooling system; Second, the treatment of compressed air part, including dryer, filter; Third, the delivery of compressed air, including gas storage tanks, pipelines, valves.
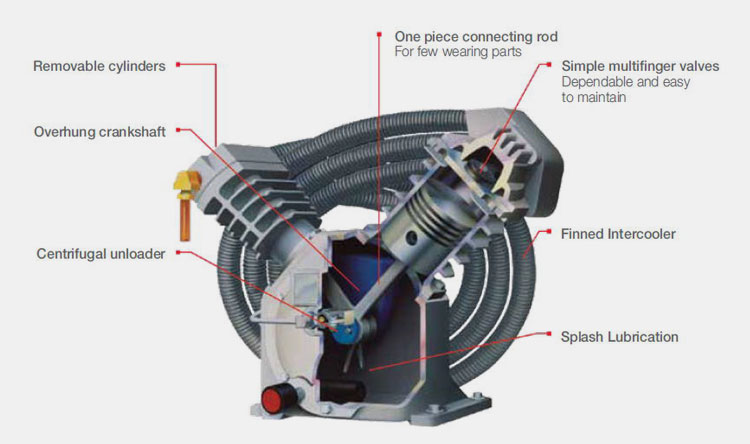
Some manufacturers suggest that some problems need to be paid attention to in the selection of air compressors. There are three commonly air compressors, piston, screw and centrifugal.
The centrifugal air compressor has a large capacity and is prone to surge when the air volume is small, so it is not suitable for pharmaceutical manufacturing companies.
Piston-type air compressors have large structural dimensions, many vulnerable parts, large noise and vibration, and low automation level, so they are rarely used by pharmaceutical manufacturers. The screw air compressor has a compact structure, a small footprint, only needs a light foundation, and has a high level of automatic control, so it is widely used.
In the selection of drying device, you also need to pay attention to many matters. When the pressure dew point of compressed air is greater than 3℃, the freeze dryer is usually used. This drying device has the characteristics of large flow range, continuous gas supply, stable pressure and no compressed air consumption.
When the pressure dew point is less than 3℃, the adsorption dryer or the combined freezing and adsorption drying device is used.
In terms of filter selection, operators need to consider the service life while ensuring the filter effect. It is reported that commonly used filters are pre-filter, precision filter, ultra-precision filter, activated carbon filter and sterilization filter.
The selection should also be combined according to different roles, performance and accuracy, so as to ensure the filtration effect, and consider the service life.
Compressed air, especially clean compressed air, is more and more widely used as process source gas in contact materials and products and the inner packaging containers of products, which has become an important factor directly affecting the quality of products.
The compressed air used in the pharmaceutical manufacturing industry plays an extremely important role in the quality of drugs. Therefore, the quality control of compressed air is also very important.
If you do not clear it properly, it will not only cause harm to the air source system, but also cause the following adverse effects in practice:
First, microorganisms and particles in compressed air can cause contamination of sterile products;
Second, the oil vapor mixed in the compressed air accumulates to a certain extent and will form an explosive flammable source, while the lubricating oil will form an organic acid after vaporization, which is easy to corrode the inner surface of the compressed air pipeline and the pneumatic components;
Third, the mixed tiny particles (such as dust and rust, etc.) are easy to damage the pneumatic components, block the throttle hole, and more seriously, cause serious pollution to the materials;
Fourth, the water mixed in the compressed air will saturate and precipitate water droplets at a certain temperature and pressure. When the compressed air is in contact with the material, it is easy to cause a serious impact on the quality of the material;
Fifth, the temperature of compressed air is too high and water is produced, which has absolute harm to vaccine and vaccine production.
Therefore, there is no certainty about compressed air in pharmaceutical applications, and the gas quality required in different production processes is not the same, but minimum standards must be guaranteed.
At present, drug safety is still a problem that cannot be ignored. Compressed air in the pharmaceutical industry plays an extremely important role in the quality of drugs.
For many problems widely existing in the design, construction and use management, the relevant departments should pay great attention to them, and gradually standardize their behavior according to the actual situation of each link.
In addition, with the improvement of automation level and the realization of unmanned operation, the biggest pollution source of compressed air will be eliminated, and the sterile effect of clean room can be fundamentally guaranteed.
Otherwise, further optimization of the compressed air system in the process of drug production, efficient clean transformation and improvement of its operation efficiency will become an important work, which has huge economic and social benefits.
Don't forget to share this post!
Tablet Press Machine Related Posts
Tablet Press Machine Related Products
Tablet Press Machine Related Videos
CONTACT US
Tell us your raw material and project budget to get quotations within 24 hours.
WhatsApp Us: +86 189 7157 0951
Want the best price & newest pharmaceutical machinery buying guide,tips and trends sent straightly to your box?Sign up for Aipak’s monthly newsletter,we’re free for your consultation and Offer you the most suitable solutions!
The Buyer's Guide
- Capsule Filling Buyer's Guide
- Blister Packaging Buyer's Guide
- Tablet Counting Buyer's Guide
- Tube Filling Buyer's Guide
- Cartoning Buyer's Guide
- Gummy Making Buyer's Guide
- CO2 Extraction Buyer's Guide
- Empty Capsules Buyer's Guide
- Suppository Filling Buyer's Guide
- Tablet Coating Buyer's Guide
- Tablet Press Buyer's Guide
- Softgel Encapsulation Buyer's Guide
Most Popular
- 7 Importance Of Pharmaceutical Packaging In Different Applications You Must Know
- 6 Advantages You Must Know About Tablet Counting Machine
- 8 Advantages of Blister Packaging You Must Know
- 6 Critical Applications of Automatic Capsule Filling Machine
- 6 Stations You must Know to Improve the Filling Quality of Automatic Capsule Filling Machine