How Much Do You Know About Solid Preparation?
There are many kinds of drugs. If classified by physical state, they can be divided into solid, semi-solid, liquid and gas dosage forms. Among them, the solid preparations accounted for 70% of all drugs, as the main form of the drug, it has good physical and chemical stability, low manufacturing cost, taking with the advantages of easy to carry,
Pharmaceutical solid preparations take powder as the starting material.In order to guarantee product quality and production process of solid preparations smoothly.
It is often necessary to process and handle drugs, including crushing, grading, mixing, granulation, drying, tablet pressing, each step of the unit operation is permeated with the application of powder technology.
It is understood that in the past, the traditional granulating process is generally used is "mixed stirring - swinging pelleting - oven drying", the disadvantage of this process is weak production capacity, cumbersome operation, and prone to cross contamination, affecting the quality and safety of products.
In order to strengthen the supervision and management of solid preparaations production and solid preparations’ quality and ensure patient medication safety, China implemented the drug production quality management practices (GMP) certification syste.
In this system, the design of the plant and equipment configuration, water, ventilation and air conditioning system, storage conditions, the file system, production management and quality management in such aspects as organization is facing more strict requirements, They also include requirements to prevent cross-contamination. It also facilitates the production of solid preparations
Under this background, the traditional craft of granulating obviously can't satisfy the conditions of authentication company, gradually become unpopular, and a new type of wet granule - boiling dry "" efficient production processes were born.
Its obvious features: high efficiency, short cycle, and conform to GMP requirement, this new type granulating process by drug companies is widely recognized and applied.It is also widely put into the production of solid preparations.
The manufacturing technique of "High Efficiency wet granulation - Boiling Drying" includes high efficiency wet granulation and boiling dry two parts, which refers to the high efficiency wet granulation is in the same equipment container can be mixed at the same time - humidification - mixing - high speed cutting granulation process such as granulation method.
Its characteristics are to avoid cross contamination, easy to operate, reduce labor intensity, in line with GMP requirements; Boiling drying, is the dry granular material in the clean hot air flow in a boiling state, through the heat exchange of hot air and material, material fast drying.
In this way, the high efficiency wet granulation machine seems to be a machine tailored for solid preparations production.
It is worth mentioning that although the advantages of the new granulation process are prominent, the key to the normal use of the new process equipment is the key to the success or failure of debugging.
Many customers reported that the above wet granulation and boiling drying equipment in the process of debugging often appear "more material head" and other quality problems, seriously affect the production of solid preparations
Granulation, as an important process in the production of solid preparations, is indispensable. After you have the pharmaceutical equipment for the production of solid preparations, how can you ensure the quality of solid preparation products?
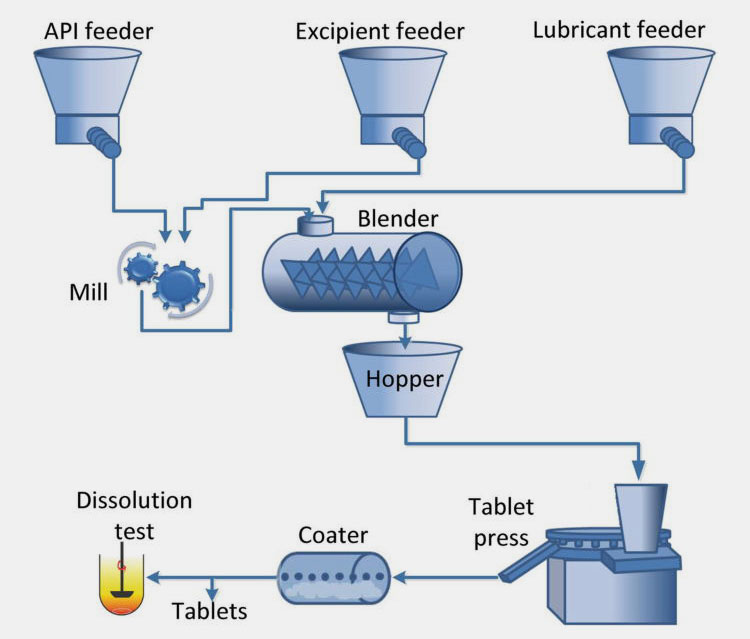
Take the preparation process of solid preparation as an example, which is a solid preparation made by uniformly pressing drugs and appropriate excipients. The preparation process of solid preparation can be divided into three steps: material filling, compression and pushing.
In order to ensure the quality of tablets, it is generally necessary to consider the influence of powder properties of tablet materials on tablet formability in prescription design and auxiliary material screening, including the analysis of tablet material fluidity, compression formability and quantification.
With the development of new auxiliary materials and high efficiency tablet pressing equipment, tablet production has entered an era of high efficiency, energy saving and high quality.
In the tablet pressing, according to the different properties of the powder, the granulation and tablet pressing method or direct tablet pressing method is generally adopted.
Among them, granulation and tablet pressing method can be divided into wet granulation and dry granulation and tablet pressing method.
Themethod of dry granulation is to press the mixture of raw materials into thin or large sheets and then crush the mixture to granulate.
After granulation, the fluidity and compression formability can also be significantly improved, which is mainly an effective method to take when the drug needs to be granulated.
However, this method has high requirements on the properties of the excipients and powders. The excipients should have good plastic deformation, good compression formability or the role of dry binder.
Otherwise, it is easy to be crushed into powder and the yield of particles is not high when crushed and granulating after tablet pressing.
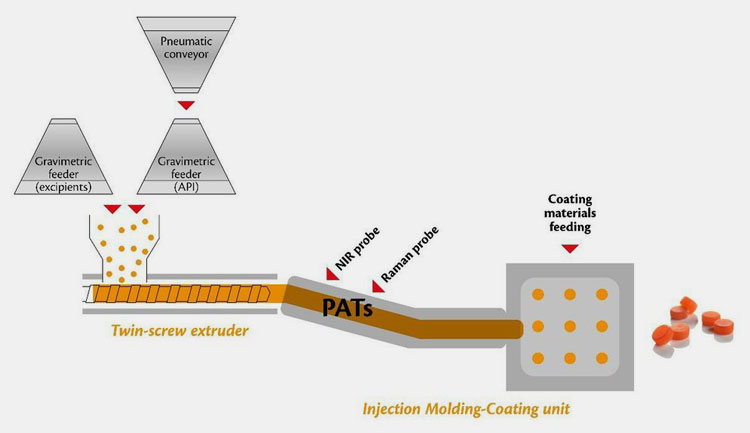
In recent years, with the continuous development of modern science and technology, as well as the promotion of GMP standardization and QbD concept, powder treatment methods continue to penetrate into the preparation process of solid preparations.
At the same time, due to the pharmaceutical technology and pharmaceutical equipment directly affect the quality of pharmaceutical products, so under the background of the current pharmaceutical industry's increasingly high requirements for pharmaceutical products, upgrading pharmaceutical technology and improving pharmaceutical equipment has become a trend.
From the trend point of view, the industry prefessionals believe that the continuous production mode is the development mode of many solid preparation companies to explore and practice at present.
From the raw material put into production to the finished product, according to the technological requirements, each process is carried out in sequence. It is expected that this model in the next five years or so will become a development direction of the industry need to study.
In general, under the backfround of aging and consumption upgrading, pharmaceutical solid preparation market demand will also continue to grow.
At the same time, with the tightening of industry supervision and the continuous improvement of quality requirements, the production process and production equipment related to solid pharmaceutical preparations also need to keep pace with the times and constantly upgrade to meet the production requirements of higher quality products.
Finally, enhance the competitive advantage in the field of solid preparations, create a competitive solid preparations overall solution and build a smart pharmaceutical factory.
Don't forget to share this post!
Tablet Press Machine Related Posts
Tablet Press Machine Related Products
Tablet Press Machine Related Videos
CONTACT US
Tell us your raw material and project budget to get quotations within 24 hours.
WhatsApp Us: +86 189 7157 0951
Want the best price & newest pharmaceutical machinery buying guide,tips and trends sent straightly to your box?Sign up for Aipak’s monthly newsletter,we’re free for your consultation and Offer you the most suitable solutions!
The Buyer's Guide
- Capsule Filling Buyer's Guide
- Blister Packaging Buyer's Guide
- Tablet Counting Buyer's Guide
- Tube Filling Buyer's Guide
- Cartoning Buyer's Guide
- Gummy Making Buyer's Guide
- CO2 Extraction Buyer's Guide
- Empty Capsules Buyer's Guide
- Suppository Filling Buyer's Guide
- Tablet Coating Buyer's Guide
- Tablet Press Buyer's Guide
- Softgel Encapsulation Buyer's Guide
Most Popular
- 7 Importance Of Pharmaceutical Packaging In Different Applications You Must Know
- 6 Advantages You Must Know About Tablet Counting Machine
- 8 Advantages of Blister Packaging You Must Know
- 6 Critical Applications of Automatic Capsule Filling Machine
- 6 Stations You must Know to Improve the Filling Quality of Automatic Capsule Filling Machine