Growth Of Neurotherapeutic Market
In recent years, the market for neurological drugs is seeing further growth due to various influences. For example, the sales revenue of central nervous system drugs in China has increased from 144 billion yuan in 2015 to 204.3 billion yuan in 2019, with a compound annual growth rate of about 9%, and it is expected that this drug market is expected to reach 250.9 billion yuan by 2024.
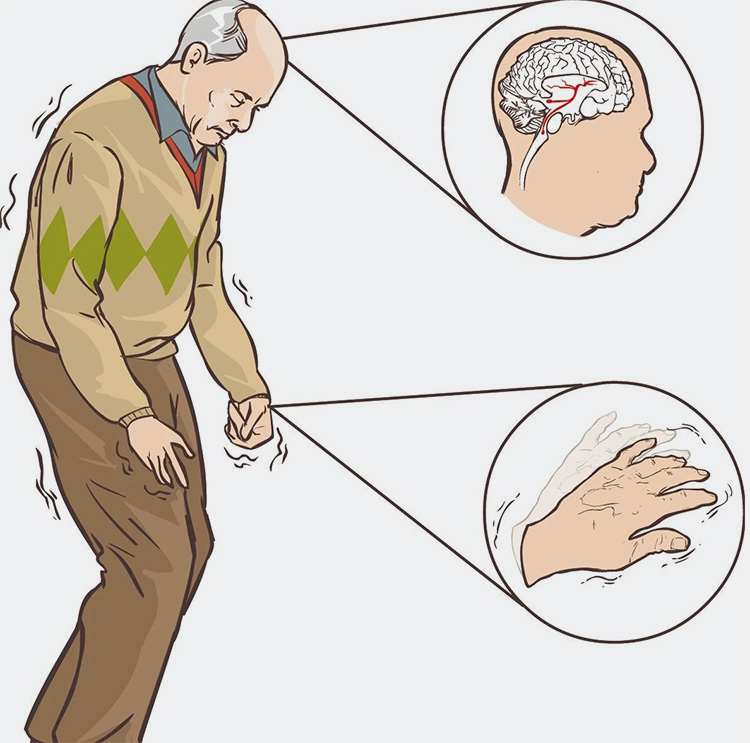
The industry said that the neurological drug segment will usher in a new situation as new moves by various companies unfold. It is understood that two of the hotter research directions in the field of neurotherapy: Alzheimer's disease (AD) and Parkinson's disease (PD), have actually begun to usher in a large number of drug companies layout at present, competing for the track.
The AD market continues to be hot, and a large number of companies are accelerating the layout
In Alzheimer's disease (AD), according to the World Alzheimer's Disease 2018 Report, there is a dementia patient about every 3 seconds globally, and there are currently at least 50 million dementia patients worldwide, and the number is expected to reach 150 million in 2050; among them, about 60%-70% are AD patients.
Currently, Eli Lilly is one of the companies very heavily invested in Alzheimer's disease (AD), and Eli Lilly's major R&D spend for 2021 relates to two research projects, one of which is the Alzheimer's treatment donanemab, a monoclonal antibody targeting N3pG for the treatment of early stage Alzheimer's disease, which has received US FDA Breakthrough It has received Breakthrough Therapy Designation (BTD) from the US FDA and the company has now submitted a rolling Biologics License Application (BLA) to the FDA seeking accelerated approval for the drug.
In 2021, Roche is also focusing on increasing its investment in neuroscience projects, including RG1450 for Alzheimer's disease (AD), a fully humanised IgG1 monoclonal antibody for subcutaneous injection, which binds and neutralises the monoclonal antibody associated with AD. It binds and neutralises the aggregation of beta-amyloid associated with AD, which interferes with the normal function of brain cells when it accumulates as plaques in the brain. Roche is currently conducting a clinical phase III trial of Gantenerumab.
In China, anti-AD drugs have also become a popular track for drug companies to develop. At present, a number of domestic pharmaceutical companies including Green Valley Pharmaceutical, Dongyang Pharmaceutical, Haizheng Pharmaceutical and Tonghua Jinma are accelerating the development of new drugs as well as generic drugs for Alzheimer's disease.
Among them, it is worth mentioning that in November 2019, GV-971 (Ganut sodium capsule, trade name "Nine Phase I"), developed by Ocean University of China, Shanghai Institute of Pharmaceutical Sciences and Shanghai Lvgu, was approved for conditional marketing, becoming the first new drug targeting the brain-intestinal axis for the treatment of Alzheimer's disease, which is original in China. Alzheimer's disease (Alzheimer's disease).
In addition, in order to further alleviate the burden of Alzheimer's disease patients in China, drug companies are accelerating the development of new drugs to provide patients with more choices outside of the country, while the country is also accelerating the entry of AD drugs into medical insurance. On December 3, 2021, the National Health Insurance Bureau announced the results of the 2021 National Health Insurance Drug Catalogue adjustment, a total of 74 drugs were added to the catalogue, and the new Alzheimer's disease drug "Nine Phase I", which has been attracting much attention, was among them.
More than 50 drugs in the PD market have been approved for marketing
Parkinson's disease (PD) is the second most common neurodegenerative disease in the world, after Alzheimer's disease. According to 2018 data, approximately 10 million people worldwide suffer from PD, and the number is rising. In recent years, based on the huge patient population and treatment demand, many pharmaceutical companies have consequently started to layout this field.
For example, in 2021, Novartis invested US$1.5 billion in a collaboration with Belgian pharmaceutical company UCB to develop two anti-Parkinson's disease (PD) drugs, UCB0599 and UCB7853, of which UCB0599 is a small molecule alpha-haptoglobin misfolding inhibitor currently in Phase II clinical trials。
In March 2022, Yixi Biologics also signed an exclusive licence agreement with BRITANNIA UK, a subsidiary of STADA Group, to acquire the rights to develop and commercialise the latter's subcutaneous injection of apomorphine for the treatment of PD in mainland China, Hong Kong and Macau.
At present, with the efforts of many pharmaceutical companies, more than 50 PD drugs have been approved for marketing worldwide. Depending on the mechanism of action, the marketed PD drugs can be broadly classified into six major categories: anticholinergic drugs, dopamine-promoting drugs, dopamine replacement drugs, dopamine receptor agonists, B-type monoamine oxidase inhibitors and catechol-O-methyltransferase (COMT) inhibitors.
Don't forget to share this post!
Capsule Filling Machine Related Posts
Capsule Filling Machine Related Products
Capsule Filling Machine Related Videos
CONTACT US
Tell us your raw material and project budget to get quotations within 24 hours.
WhatsApp Us: +86 189 7157 0951
Want the best price & newest pharmaceutical machinery buying guide,tips and trends sent straightly to your box?Sign up for Aipak’s monthly newsletter,we’re free for your consultation and Offer you the most suitable solutions!
The Buyer's Guide
- Capsule Filling Buyer's Guide
- Blister Packaging Buyer's Guide
- Tablet Counting Buyer's Guide
- Tube Filling Buyer's Guide
- Cartoning Buyer's Guide
- Gummy Making Buyer's Guide
- CO2 Extraction Buyer's Guide
- Empty Capsules Buyer's Guide
- Suppository Filling Buyer's Guide
- Tablet Coating Buyer's Guide
- Tablet Press Buyer's Guide
- Softgel Encapsulation Buyer's Guide
Most Popular
- 7 Importance Of Pharmaceutical Packaging In Different Applications You Must Know
- 6 Advantages You Must Know About Tablet Counting Machine
- 8 Advantages of Blister Packaging You Must Know
- 6 Critical Applications of Automatic Capsule Filling Machine
- 6 Stations You must Know to Improve the Filling Quality of Automatic Capsule Filling Machine