Why Powder Feature Matters In Development And Preparation Of Solid Dosage Forms?
Your medicines are made of various powder, do you know that? Do you know that the different powder feature make different affect for your medicine products. Besides your medical tablets, there are also the effect for your powder material on solution, injection and other oral products.
How to control powder quality during solid dosage forms manufacturing? How to deal with excipients powder for your solid dosage form? What process your powder may experience in solid dosage form products manufacturing? Why powder feature matters in development and preparation of solid dosage forms? Come on and get answers here!
1.What Is Solid Dosage Forms?

Solid Dosage Form-Sourced:pharma-trends
Solid dosage form is the drug which is made with solid state for medicine use. There are tablets, pills, capsules, granules, powder and so on. Most solid dosage forms are swallowed and dissolved by your stomach. You may find the solid dosage forms for various medicine types and brands.
Solid dosage form has the wide application for its stable feature, easier formulating and better packaging and transporting. The oral administration way is more safe and welcomed. That may be the reason for the lasting welcome of your solid dosage forms.
2.WhatIs Powder And The Basic Feature Of Powder?
Medicine Powder-Sourced:373lab
Powder is an aggregate of countless solid particles. Its microscopic properties mainly refer to the characteristics of individual particles that make up the powder, such as particle size, shape, specific surface area, surface energy, surface roughness, and particle size distribution. These properties directly affect the flow of the powder and determine its other characteristics.
The macroscopic properties of a powder refer to its characteristics as a whole, such as bulk density and porosity, hygroscopicity, wettability, cohesiveness, flow and fillability, compressibility, and form. These macroscopic properties are influenced by the microscopic ones and are closely related to the manufacturing process of formulations. By adjusting the microscopic properties through formulation techniques, product quality control can be achieved.
3.What Are The Powder Characteristics During Solid Dosage Forms Manufacturing?
Solid Dosage Forms-Sourced:cargill
What are the powder characteristics during solid dosage forms manufacturing? The study of powder properties plays a crucial guiding role in the development of solid dosage forms. Below are the correlation between the powder characteristics and the quality attributes of pharmaceutical formulations.
| Quality Attribute of the Formulation | Relevant Powder Property Affecting Product Quality | Degree of Influence |
| Appearance | Formability | Moderate |
| Tablet weight variation | Flow and fillability, particle size distribution | |
| Hardness | Compressibility | Important |
| Friability | Compressibility | |
| Moisture content | Hygroscopicity | |
| Dissolution rate | Particle size distribution | |
| Assay (content) | Density and porosity, flow and fillability, particle size distribution | Very Important |
| Content uniformity | Particle size distribution, flow |
4.What Industry Will Apply Powder For The ManufacturingBesides Pharmaceutical Industry?
Pharmaceutical industry makes the great applying of powder for the various medicine manufacturing. And here are also some industries which will apply powder for the manufacturing.
Food industry
Food Industry-Sourced:hanningfield
Food industry makes the wide applying of powder for its various food products manufacturing. Fine and even powder is the prove of the high qualified food products. You may find the use of powder for cookie, cake, noodle and many food products for manufacturing.
Chemical industry
Chemical Industry-Sourced:freepik
There are various chemical products and for different chemical products, the request on high qualified and high standard powder is required. You may find the use of chemical powder for cleaning agent, ceramic products, cloth products, plastic products and so on.
Agricultural industry
For agricultural industry, you may find the applying of powder products for various agricultural products. For fertilizer, pesticides and so many agricultural products, you may find the wide applying of powder. Even and uniform powder is the base for the high qualified agricultural products.
Cosmetic industry
Cosmetic Industry-Sourced:ewg
Cosmetic products make the high request for the powder feature and quality which is applied for cosmetic products manufacturing. The fine and even powder is the prove of the high qualified cosmetic products. Cosmetic industry makes thus also the wide use of powder in its industry.
5.How To Control Powder Quality During Solid Dosage Forms Manufacturing?
The high qualified powder products is the base of your high qualified medicine products. Do you know the way for the controlling of powder quality during solid dosage form manufacturing?
Powder Quality-Sourced:con-cret
Establishing particle size standards
Particle size is a critical property of powders and can significantly affect solubility, density, and flow. For solid dosage forms, particle size standards must be established if the particle size of the active pharmaceutical ingredient (API) impacts dissolution rate, solubility or bioavailability, manufacturing process, stability of the formulation, content uniformity, product appearance. Reducing the particle size of poorly soluble drugs is an effective way to improve dissolution rate, dissolution speed, and bioavailability.
Storage conditions
After milling, APIs may undergo powder agglomeration during long-term storage. This can lead to a reduction in dissolution performance and discrepancies between the dissolution profile of the test product and the reference product.
Uniform particle size
Particle size can also affect the manufacturing process of the formulation. Since the initial particle size of the API influences its mixing uniformity with excipients, large particle size differences can lead to segregation.
Particle size affects the melting rate when preparing solid dispersions. Moreover, the particle size distribution of the intermediate solid dispersion should be defined according to the needs of the next manufacturing step.
6.How To Deal With Excipients Powder For Your Solid Dosage Form?
Pharmaceutical excipients are directly involved in the processes of drug preparation molding, disintegration, dissolution, sustained release and controlled release, which will affect the quality, safety and effectiveness of drugs. Therefore, determining the particle properties of excipients is of great significance to ensure the quality of preparations.
Excipients Powder-Sourced:indiamart.
Excipients powder function
In pharmaceutical formulations, excipients are typically used for their specific functional properties. Assessing, classifying, and setting acceptable limits for these functional attributes is essential to ensure formulation quality.
For example, particle size is one such functional property. The particle size of diluents can influence the form of solid dosage forms. Different grades of mannitol, for instance, exhibit varying powder characteristics, including particle size, flow, and crystallinity. These differences can directly affect the quality of the final product.
Excipients powder feature
When it comes to excipients control, applicants should evaluate the impact of excipients from different sources, batches, and grades on both the quality of the formulation (e.g., dissolution rate, content uniformity) and the manufacturing process.
It is not sufficient to rely solely on compliance with general standards. Instead, functional attributes of excipients—such as particle size and bulk density—should be defined based on the specific requirements of the dosage form, and appropriate in-house control standards should be established. This ensures the smooth and consistent production of the pharmaceutical formulation.
7.What Process Your Powder May Experience In Solid Dosage Form Products Manufacturing?
Powder experience the long manufacturing road for the qualified and reliable medicine products forming and manufacturing. What process your powder may experience in solid dosage form products manufacturing?
Granulation process
Granulation Process-Sourced:foodfeedfinechemicals
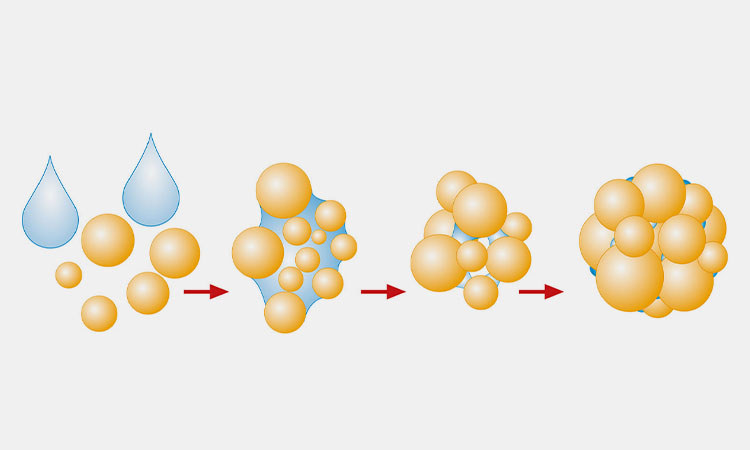
The granulation process can effectively improve particle properties. By increasing particle size, it enhances flow, and can also improve compactability, fillability, and compressibility, while preventing segregation of components after mixing.
Additionally, properties such as particle density and particle size distribution can significantly influence key quality attributes of solid dosage forms, including content uniformity and dissolution rate.
Mixing process
Mixing Process-Sourced:wikipedia
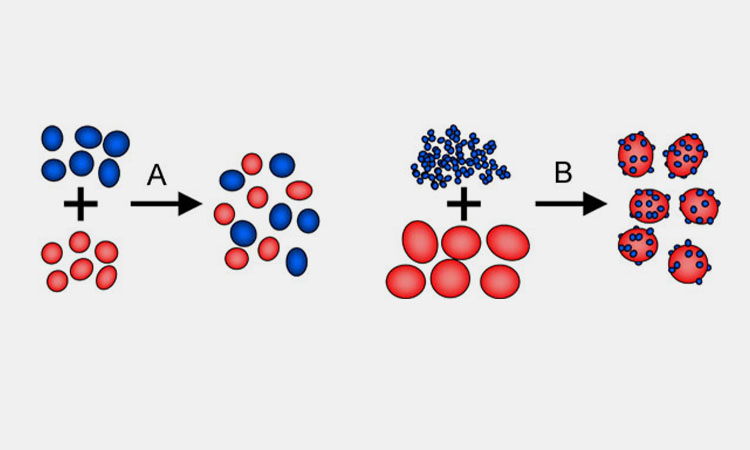
In the manufacturing process of solid dosage forms, material mixing is a critical unit operation, as the uniformity of the mixture directly determines product quality.
Due to differences in particle size, shape, bulk density, flow, electrostatic properties, surface energy, and moisture content among the various components, incomplete or non-uniform mixing may occur. This can negatively impact not only the final product quality such as content uniformity, but also the stability and consistency of the manufacturing process.
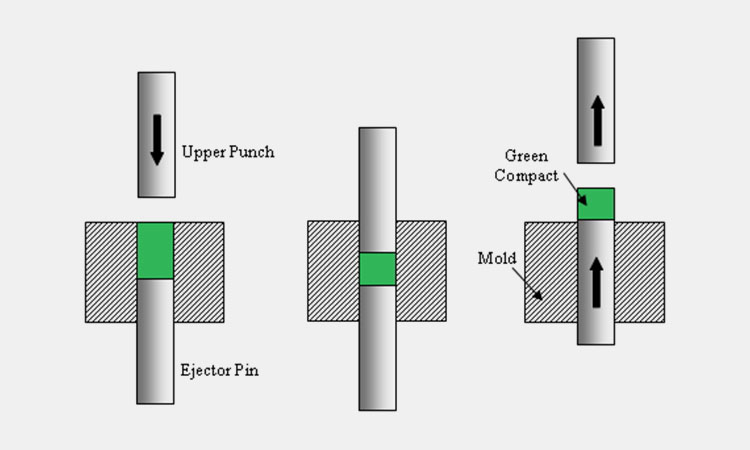
Pressing or filling process
Pressing Or Filling Process-Sourced:researchgate
During the formulation and shaping stages of drug production, maintaining the favorable powder properties of materials from earlier processing steps becomes especially important. This is due to factors such as material storage, transport between processes and locations, and vibrations from production equipment.
Powder properties like flow, uniformity of particle size distribution, and compressibility are critical to product quality, as they influence formulation attributes like tensile strength and content uniformity.
Evaluating whether the drug powder is uniformly mixed is a direct way to detect potential powder segregation, and it serves as an important process control strategy during pharmaceutical manufacturing.
8.Why Powder Feature Matters In Development And Preparation Of Solid Dosage Forms?
Powder feature makes the important work for the developing and preparing of solid dosage forms for your medical business.
Powder Feature-Sourced:shunnarah
The properties of powders, especially their flow, are of great importance in the development and preparation of solid dosage forms. Understanding and mastering the powder characteristics of materials can help solve issues related to formulation selection, process improvement, quality control, and production.
Approximately 80% of solid dosage forms and their manufacturing involve powdered components. Therefore, many pharmaceutical processes—such as blending, transfer, storage, feeding, compression, and fluidization—involve powder handling.
Powder properties, particularly flow, play a key role in the development and manufacturing of solid dosage forms, as the flow behavior of materials may influence the choice of excipients and production processes. Thus, recognizing and mastering the powder-related properties of materials contribute to addressing challenges in formulation screening, process optimization, quality assurance, and manufacturing.
9.What Are The Machines Applied In Solid Dosage Forms Manufacturing?
What are the machines applied in solid dosage forms manufacturing? Here are the common machines which are applied for solid dosage forms manufacturing.
Pharmaceutical pulverizer machine
AIPAK Crusher Machine
| Input Process Parameters | Pharmaceutical pulverizer machine speed, screen mesh size |
| Input Material Properties | 1.Active pharmaceutical ingredient (API): particle size, crystal form, moisture content, viscosity, stability; 2. Excipients: particle size and distribution, viscosity, moisture content |
| Production Process | Pre-treatment of excipients |
| Output Material Properties | 1. API: particle size and distribution, crystal form, flowability, viscosity, moisture content, hygroscopicity, etc.
2. Excipients: flowability, viscosity, moisture content, particle size and distribution |
Granulator machine
AIPAK Granulator Machine
| Input Process Parameters | Stirring paddle speed, chopper speed, premixing time, binder addition time and method, granulation time, etc. |
| Input Material Properties | From previous process: 1. API: particle size, crystal form, flow, viscosity, moisture content
2. Excipients: flow, viscosity, moisture content, particle size and distribution 3. Binder type and amount |
| Production Process | Wet granulation of granulator machine |
| Output Material Properties | Granule compactness, size, stickiness, moisture, etc. |
Oscillating granulator
ALLPACK Oscillating Granulator
| Input Process Parameters | Screen mesh size, granulating blade speed of oscillating granulator |
| Input Material Properties | Granule size, compactness, stickiness from previous step |
| Production Process | Wet sizing |
| Output Material Properties | Wet granule size, compactness, particle distribution, etc. |
Fluid bed dryer
AIPAK Fluid Bed Dryer
| Input Process Parameters | Inlet air volume, temperature, humidity, back-blowing frequency, etc. |
| Input Material Properties | Granule size and compactness from previous step |
| Production Process | Fluid bed drying of fluid bed dryer |
| Output Material Properties | Granule size and distribution, bulk density, moisture, etc. |
Lifting granulator
Lifting Granulator-Sourced:wonsen
| Input Process Parameters | Screen mesh size, granulating blade speed |
| Input Material Properties | Granule size and distribution, bulk density, moisture from previous step |
| Production Process | Dry sizing |
| Output Material Properties | Particle size and density distribution, moisture, bulk density, flow (e.g., angle of repose, Carr index, etc.) |
Bin blender
AIPAK Bin Blender
| Input Process Parameters | Mixing speed and time, order of addition, type of agitator |
| Input Material Properties | From previous step: particle size and distribution, moisture, bulk density, flow, and properties of added excipients: density, particle size and distribution, bulk density, moisture, flow, etc. |
| Production Process | Final blending from bin blender |
| Output Material Properties | Uniformity of granule content, particle size and distribution, density, bulk density, moisture, flow, etc. |
Tablet press machine
AIPAK Tablet Press Machine
| Input Process Parameters | Type of tablet press, compression force (pre/main), tablet speed, feed speed and method (e.g., force feeder), hopper fill level |
| Input Material Properties | From previous step: uniformity of granule content, particle size and distribution, bulk density, moisture, compressibility, flow |
| Production Process | Tableting of tablet press machine |
| Output Material Properties | Tablet surface smoothness, content, content uniformity, dissolution, weight variation, friability, hardness, etc. |
10.Why You Should Apply Pharmaceutical MachinesFor The Raw Powder Material Dealing For Solid Dosage Form?
Why you should apply pharmaceutical machines for the raw powder material dealing for solid dosage forms? Here are the reasons you may check.
High efficiency
High Efficiency-Sourced:laars
Pharmaceutical machines make the high efficient job for the raw powder material dealing for solid dosage form. The machinery job from your pharmaceutical machines can improve the efficiency of your manufacturing process with high speed. With pharmaceutical machines, you may have get your high qualified solid dosage form.
High accuracy
For medicine products, there is the precise request on the medicine dosage, manufacturing and so on. With pharmaceutical machines, you may get the medicine products with high accuracy. And the high accurate solid dosage form is the medicine which can make the efficient and reliable medicine products.
Hygiene prove
With the little adding of labor in the solid dosage form manufacturing process, you can have the hygiene condition proved from the pharmaceutical machines in solid dosage manufacturing. From pharmaceutical machine, you may have the hygiene of your solid dosage form proved.
Quality prove
Quality Prove-Sourced:strategiatx
You may have the quality proved with your pharmaceutical machines for your solid dosage forms manufacturing. The outstanding pharmaceutical machine work can prove the high quality of your solid dosage forms of your products. Quality-proving is the important thing pharmaceutical machine can prove you.
Lower cost
The purchase for your pharmaceutical machine may be big. But in the long run, pharmaceutical machine can make great saving for your pharmaceutical business. Besides, the great qualified solid dosage forms and high efficient manufacturing process make the great benefits for your business.
11.Are There Any Other FactorsAffect The Solid Dosage Forms Manufacturing?
Powder feature and pharmaceutical machines make the important work for your manufacturing of solid dosage forms. Are there any other factors affect the solid dosage forms manufacturing?
Formulation
Formulation-Sourced:iptonline
Different formulation of different solid dosage form makes the different manufacturing affect your solid dosage medical products. Even and uniform formulation makes the complete medicine structure. The proportion of raw medicine powder and excipients powder makes the different formulation type.
Manufacturing environment
Manufacturing Environment-Sourced:pharmaron
Different temperature and humidity make the different affect for your solid dosage form manufacturing. Manufacturing environment makes the different affect for your powder flow, compressibility and stability. You should thus adjust the environment condition for the suitable solid dosage form manufacturing.
Operator skill
The difference operation skill and various proficiency make also the different work for your solid dosage form manufacturing. The proficient operator can make the reliable and efficient machine operating for your solid dosage forms. You should improve your operator skill for your great solid dosage form work.
Regulatory standard
Regulatory Standard-Sourced:fairinstitute
Different regulatory standard makes different request on your solid dosage forms. The manufacturing practice of your solid dosage form should conform to the relevant regulatory standard. The previous knowing of the regulatory standard is the base for your high qualified solid dosage forms.
Conclusion
Why powder feature matters in development and preparation of solid dosage forms? You may have your answers. For solid dosage form, the close contact of it and different powder makes the different medicine feature. For your high qualified solid dosage form, you should have a deep knowing for better work. For any problem or question, contact AIPAK now!
Don't forget to share this post!
Bin Mixer Related Posts
Bin Mixer Related Products
Bin Mixer Related Videos
CONTACT US
Tell us your raw material and project budget to get quotations within 24 hours.
WhatsApp Us: +86 181 6426 8586

Want the best price & newest pharmaceutical machinery buying guide,tips and trends sent straightly to your box?Sign up for AIPAK’s monthly newsletter,we’re free for your consultation and Offer you the most suitable solutions!
The Buyer's Guide
- Capsule Filling Buyer's Guide
- Blister Packaging Buyer's Guide
- Tablet Counting Buyer's Guide
- Tube Filling Buyer's Guide
- Cartoning Buyer's Guide
- Gummy Making Buyer's Guide
- CO2 Extraction Buyer's Guide
- Empty Capsules Buyer's Guide
- Suppository Filling Buyer's Guide
- Tablet Coating Buyer's Guide
- Tablet Press Buyer's Guide
- Softgel Encapsulation Buyer's Guide
Most Popular
- 7 Importance Of Pharmaceutical Packaging In Different Applications You Must Know
- 6 Advantages You Must Know About Tablet Counting Machine
- 8 Advantages of Blister Packaging You Must Know
- 6 Critical Applications of Automatic Capsule Filling Machine
- 6 Stations You must Know to Improve the Filling Quality of Automatic Capsule Filling Machine