How Solid Processing Equipment Affects Tablet Quality in Pharma Production?
A single finished product of solid dosage form is involved in a series of manufacturing steps and processing equipment. The primary aim is to formulate a true combination of Active Pharmaceutical Ingredients with excipients. No one can deny the fact that solid dosage formulation is the world’s most popular mode of medication. The story has not ended yet, or more growing day by day.
According to Future Market Insight, it is estimated that by 2027, its business will grow further to $926.3 billion. Therefore, more opportunities for pharma manufacturers to offer impressive medications. All that can be possible with the right machinery and a reliable supplier like AIPAK; the company has emerged with providing innovative, budget-friendly, automated solutions for solid processing.
With a series of high-performance and extensive equipment, the company ensures the right machine is the success behind effective and quality pharma production. In this article, you will explore basic objectives related to ‘How Solid Processing Equipment Affects Tablet Quality in Pharma Production’. Let’s read it out.
1.What are the Key Stages of Solid Processing in Pharmaceutical Production?
The key stages of solid processing in pharmaceutical production are described as:
Pharmaceutical Pulverizer Machine

AIPAK Pharma Pulverizer
To reduce the size of larger particles or to attain uniformity in particle sizes, a pharmaceutical pulverizer machine is used in solid processing. This machine grinds, crushes, pulverizes, or cuts solid substances into smaller fragments by exerting forces, such as impact, attrition, or compression.
Sifter Machine
AIPAK Sifter Machine
Blending large particles with fine powders is a challenging task that is encountered with the help of a sifter machine. Large lumps, agglomerates, foreign objects, and debris from raw materials are separated by this machine. It comes with interchangeable screens with different aperture sizes to sieve fine or ultra-fine particles. In pharmaceutical processing, the sifter machine also performs the function of grading or classification, in which materials are divided into various size fractions. Its types include vibro sifters and centrifugal sifters.
Bin Mixer
AIPAK Bin Mixer
A bin mixer mixes materials through the rotation and swinging motion of its detachable bin, which is the primary mixing chamber. The movement of the bin promotes mechanical mixing, allowing thorough and uniform distribution of ingredients in the mixture. Some of its types, like tanker-type mixer or double paddle tank type mixer, also include agitating shafts or paddles within the bin to handle dense or cohesive powders. Its closed design reduces contamination risk, ensuring hygienic blending.
Bin Blender
AIPAK Bin Blender
On the other hand, a bin blender or Intermediate Bulk Container (IBC) blender is a type of tumbling blender that blends materials through its controlled rotation. It features a removable bin, positioned at a slight incline of 15° or 30° relative to its axis. The materials are loaded in the bin, which rotates in three-dimensional motion, promoting continuous mixing movement. This action enhances diffusive mixing, resulting in a homogeneous final blend.
High Shear Mixer Granulator
AIPAK High Shear Mixer Granulator
Twin operations of mixing and wet granulation in the solid processing line are carried out by specialized equipment called a high shear mixer granulator. It is also termed a rapid mixer granulator, and it converts fine heterogeneous powders into high-density homogenous granules using granulating fluid. In this way, the flowability and compressibility of materials are increased for the tableting and encapsulation processes.
Fluid bed dryer
AIPAK Fluid Bed Dryer
In solid processing, drying is achieved by using a fluid bed dryer. This equipment is employed to remove moisture content from granules and powders after wet granulation. It utilizes warm air to circulate through the product bed, ensuring even drying without overheating. This helps to attain constant particle size and moisture level, which are requisite for tablet uniformity.
Tablet Coating Machine
AIPAK Tablet Coating Machine
The process of applying a thin, uniform layer of coating material on tablets is performed by a tablet coating machine. This coating protects the tablet from degradation, enhances its taste, and controls drug release in the body. Tablets are placed in a rotating perforated drum, where coating solution is sprayed on tablets and warm air is passed through them to evaporate the solvent. This process is repeated until a fine coating is formed on the tablet.
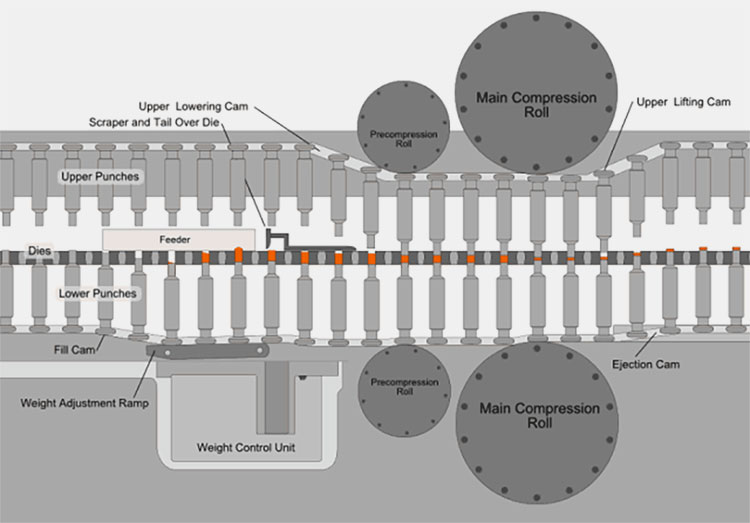
Tablet Press Machine
AIPAK Tablet Press Machine
The tablet press machine contributes to shaping the powdered drug into a solid tablet of constant size and weight. It helps to create precise tablets with a smooth surface for coating. The process takes place by loading powders or granules into a die, where upper and lower punches compress them to form a tablet. It ensures accurate dose, good hardness, and uniform tablet quality.
Capsule Polishing Machine
AIPAK Capsule Polishing Machine
The specific role of the capsule polishing machine is to remove dust, plastic fragments, or metal debris from filled capsules and improve their finishing. The process starts by feeding capsules into a rotating brush chamber. This chamber contains soft brushes that polish the capsule surface, and vacuum suction is there to collect dust and debris. In this way, clean and shiny capsules are manufactured without challenging their integrity.
Deblister Machine
AIPAK Deblister Machine
The deblistering machine allows efficient recovery of filled tablets from rejected blisters during packaging. This step enhances the productivity of solid processing and minimizes the product loss. It works by passing the blister packs between pressure rollers that apply force to push tablets out. The recovered tablets are collected separately while the empty foil is discarded.
2.How Equipment Design Influences Tablet Quality?
The equipment design influences tablet quality due to the following reasons:
Equipment Design Influences Tablet Quality
Mixing Uniformity
Mixing is the first step of tablet manufacturing, and it must be done uniformly to attain even distribution of the drug. The flow pattern of tablet ingredients can be affected by the impact of impeller design; that’s why it needs to be well-crafted. Moreover, the speed control should remain optimal throughout the mixing process, as a slight change can result in poor mixing or damaged particles. Another important thing is loading ratio of material must be relative to the capacity of the mixer so that particles get enough space for mixing and the equipment can perform efficiently.
Granulation control
Granulation control is integral for maintaining tablet quality. The role of impeller/chopper speed is to control the proper binder distribution and formation of even granules. The chopper prevents lump formation, due to which sufficient control over chopper speed shows a balance between granule size and its ability to flow. Binder is crucial for granulation, so binder addition timing must be accurate to ensure uniform moisture distribution and cohesion of the granule.
Drying Efficiency
Drying efficiency talks about how ideally moisture can be removed from wet granules without challenging their quality. The important aspect of drying efficiency is to deliver a uniform effect of air distribution through the drying equipment. In addition to this, temperature uniformity and time during the whole process are necessary to keep the drying process fast and effective. Keep in mind that proper drying time is requisite to get well-dried, flowable, and even granules.
Particle Size Control
A tablet’s quality is directly related to the particle size control, as it influences the flowing properties, dissolution rate, compressibility, and uniformity of the furnished tablet. The importance of screen type needs to be selected appropriately as particles pass through this mesh during milling or sifting. Milling is carried out to get a fine particle size distribution, so it is important to keep controlled mill speed. Moreover, suitable vibration frequency during sifting allows regular passage for particles as per their sizes, which ensures product uniformity.
3.What are the Common Tablet Quality Issues Caused by Poor Processing Control Weight variation?
Tablet quality heavily relies on effective processing and procedures. Some of the common tablet quality issues caused by poor processing are mentioned below.
Content Uniformity Failure
Weight variation in tablet-Picture Courtesy: Pharmaceutical Online
In tablet manufacturing, each tablet must contain an equal number of APIs to ensure the required uniformity. Any inconsistency may result in undermining the therapeutic effects of the tablet, which is a serious health risk. Some of the factors affecting tablet consistency are segregation of APIs and excipients due to inadequate mixing, improper transfer or handling, and differences in particle size. These issues lead to content uniformity failure, consequently posing a risk of reduced effectiveness or overdose.
Capping, Lamination, Or Cracking
Lamination of tablet-Picture Courtesy: Tableting
These are mechanical defects that usually occur due to poor compression force, improper granule moisture, and air retention. In capping, the top layer of the tablet partially or completely separates. Lamination occurs due to the presence of trapped air or improper compression force, meanwhile, cracking refers to the visible cracks that appear on the surface of the tablet which usually occur due to poor formulation or mechanical stress.
Poor Dissolution Rate
Dissolution -Picture Courtesy: AAPS
This issue occurs when the tablet fails to dissolve within the specified time, affecting the release of active pharmaceutical ingredients and other excipients of the tablet in the body. Factors affecting the dissolution rate of a tablet are poor compression force, inadequate mixing, or utilizing poorly soluble excipients. Due to improper dissolving, the drug may not reach the required amount of concentration, undermining the therapeutic effectiveness of the tablet.
Tablet Hardness Inconsistency
Tablet defect testing- Picture courtesy: AAPS
Tablet hardness refers to the mechanical strength and resistance of the tablet during exposed conditions like transportation, handling, and storage. Inconsistent tablet hardness led to high friability, variation in dissolution time, etc. Common causes that lead to tablet hardness inconsistency are improper compression force, poor powder flow, and inconsistent granule size flow. These are the parameters that affect tablet hardness inconsistency and lead to non-compliance with regulatory requirements and reduced therapeutic effect.
4.What is the Role of Automation and Sensors in Ensuring Consistency?
Working principle or tablet press machine
The automation and sensors are the important entities of any machine that help in ensuring production in a consistent manner. The following prime objectives are described below:
Precise Operational Control
Automation enhances the precise operational control over critical parameters like temperature, pressure, mixing speed, vibration frequency, etc., which helps to minimize human error and helps in production consistency.
Quick Monitoring
They help to encounter any variation in the ongoing process, ensuring uniform results and production within standardized limits.
Constant Product Quality
Automation and sensors play a key role in maintaining the uniformity in weight, coating thickness, and drying time throughout the whole production. This ensures constant product quality each time, regardless of batch size or operator.
High Productivity
All the steps are performed with perfection in less time and with reduced material waste.
Traceability And Regulatory Compliance
Automation and sensors promote quick monitoring and data recording of each batch. This ensures complete traceability and consistent regulatory compliance during the entire solid processing operation.
Predictive Maintenance
These features identify issues related to manufacturing equipment at an early stage. This enables timely predictive maintenance that improves production efficiency and reduces equipment downtime.
Sustained Environmental Control
They help achieve sustained environmental control throughout the process by maintaining optimal temperature, humidity, and air pressure.
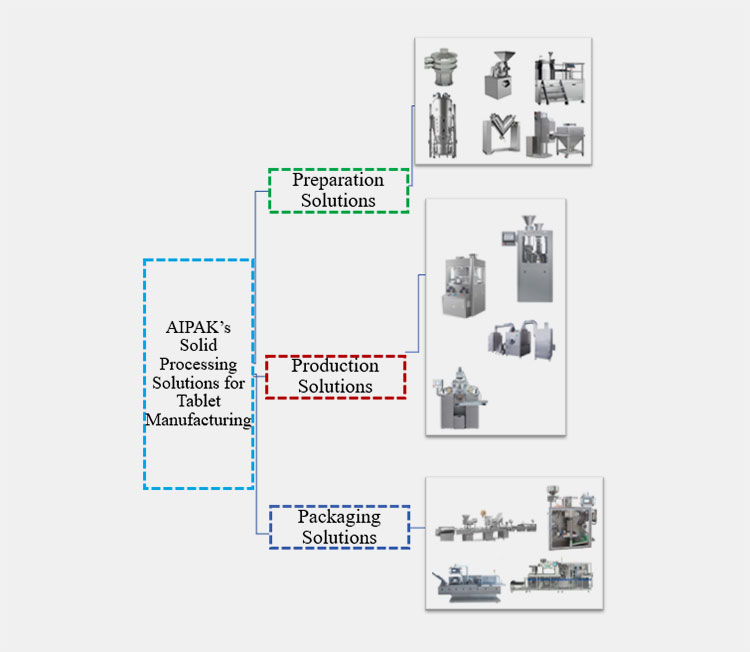
5.What are AIPAK’s Solid Processing Solutions for Tablet Manufacturing?
The AIPAK’s solid processing solutions for tablet manufacturing are discussed below:
Comprehensive Presentation of AIPAK’s Solid Processing Equipment
AIPAK is the hub of manufacturing and packaging solutions. The company covers all aspects of equipment that are required in every stage of solid formulation till its final packaging. The machinery designs are based on ‘3-P’ solutions: preparation, production, & packaging equipment.
AIPAK’s Preparation Solution
When your preparation of raw materials is done right, the next stages will be smooth and ideal. AIPAK’s preparation solutions are high-speed, automatic, and affordable equipment, such as Pulverizer, cone-mill mixers, blenders, granulators, driers, etc. They’re provided to maintain consistent and uniform outcomes for quality production of solid dosage formulations.
AIPAK’s Production Solution
For the production stage, AIPAK’s premium quality machinery includes a tablet press, coating machines, filling machines, etc. These reliable machines give up to your desirable production capacity, astringent to regulatory compliance.
AIPAK’s Packaging Solution
The extensive solutions related to packaging included counting lines, cartoning machines, blister packaging machines, strip packaging machines, etc. Therefore, by following all machines, AIPAK promises to support uniform and large-scale solid dosage production with professional and tremendous quality.
6.What are the Best Practices for Maintaining Equipment Performance?
In order to achieve effective and even tablet consistency, it is obligatory to maintain the equipment carefully. Some of the best practices that lead to effective equipment performance are as follows.
Best Practices for Maintaining Equipment Performance
Scheduled Maintenance Strategy
A proactive maintenance plan helps detect misalignment and defects before they affect the production process. Working components such as seals, dies, and punches are in constant use; therefore, their damage can cause serious operational problems. Continuous monitoring of these critical components prevents production deviations such as non-uniform tablet granulation, capping, lamination, and more.
Proper Sanitation Protocols
Residue accumulation from previous functions eventually results in cross-contamination, weight variation, and spoilage, which compromises product quality. In order to avoid infiltration in equipment, it is essential to integrate cleaning and sanitation protocols as well as disassemble of equipment when required.
Frequent Calibration of Parameters
To ensure optimal performance of pharmaceutical equipment involved in key processes such as compression, mixing, and drying, it is important to regularly calibrate the equipment to avoid any operational risk and non-compliance with regulatory requirements. In case these parameters are not frequently maintained, functional challenges such as incorrect drying temperature, inadequate compression force, as well as improper mixing speed are most likely to occur, which could hinder process stability.
Effective Documentation
For effective equipment performance, proper documentation of cleaning logs, calibration records, and maintenance activities is essential. Documentation of these parameters not only adheres to regulatory compliance, but it also helps operators conduct trend analysis to detect potential issues and damages.
Conclusion
The solid processing equipment is a strong backbone of pharma production. The excellent setup ensures production quality at a high level. Whether it is preparation, formulation, or packaging, the processing equipment should uphold the international standard and required working features. Using high-performance equipment like AIPAK machinery can help in producing defined medication batches and maintaining an ideal workflow. Remember, a minor mistake can affect the overall efficacy of a solid dosage formulation. Therefore, invest in the right machine with the right supplying partner like AIPAK. To know our reliable and cost-effective solid processing solutions, please contact AIPAK’s technical team now.
Don't forget to share this post!
Tablet Press Machine Related Posts
Tablet Press Machine Related Products
Tablet Press Machine Related Videos
CONTACT US
Tell us your raw material and project budget to get quotations within 24 hours.
WhatsApp Us: +86 156 0710 8630

Want the best price & newest pharmaceutical machinery buying guide,tips and trends sent straightly to your box?Sign up for AIPAK’s monthly newsletter,we’re free for your consultation and Offer you the most suitable solutions!
The Buyer's Guide
- Capsule Filling Buyer's Guide
- Blister Packaging Buyer's Guide
- Tablet Counting Buyer's Guide
- Tube Filling Buyer's Guide
- Cartoning Buyer's Guide
- Gummy Making Buyer's Guide
- CO2 Extraction Buyer's Guide
- Empty Capsules Buyer's Guide
- Suppository Filling Buyer's Guide
- Tablet Coating Buyer's Guide
- Tablet Press Buyer's Guide
- Softgel Encapsulation Buyer's Guide
Most Popular
- 7 Importance Of Pharmaceutical Packaging In Different Applications You Must Know
- 6 Advantages You Must Know About Tablet Counting Machine
- 8 Advantages of Blister Packaging You Must Know
- 6 Critical Applications of Automatic Capsule Filling Machine
- 6 Stations You must Know to Improve the Filling Quality of Automatic Capsule Filling Machine